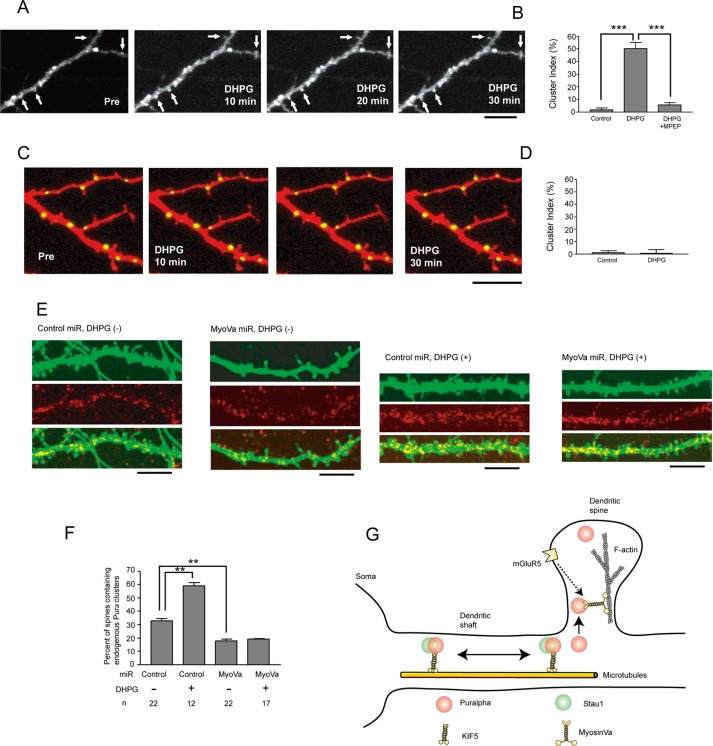
FIGURE 3:
(A) Time-lapse recording of YFP-Puralpha clusters after DHPG treatment reveals that YFP-Puralpha accumulates within spines. Scale bar: 10 μm. (B) The average cluster index is increased 30 min after treatment with 100 μM DHPG (22 clusters from 12 neurons from two mice were examined). The DHPG-dependent spine accumulation is abolished by MPEP, an mGluR5 antagonist (18 clusters from 11 neurons from two mice were examined). For the control, 22 clusters from 12 neurons from two mice were examined. Error bars indicate SEM. ***, p < 0.001; Student’s t test. (C) Time-lapse recording after DHPG treatment reveals that Stau1-GFP clusters remained in dendritic shafts. Scale bar: 10 μm. (D) The average cluster index is not increased 30 min after treatment with DHPG (12 clusters from five neurons from two mice were examined). For control, 12 clusters from six neurons from two mice were examined. Error bars indicate SEM. (E) Forty-eight hours after transfection with miR vectors, neurons (15 DIV) were treated with DHPG for 1 h, fixed, and stained with anti-Puralpha antibodies. Scale bars: 10 μm. (F) Statistical analysis of E. Percentage of spines containing endogenous Puralpha clusters was increased by DHPG treatment and was decreased by myosin Va silencing. Error bars represent SEM. **, p < 0.01; Student’s t test. (G) Schematic model. Puralpha clusters are localized in both dendritic spines and shafts, whereas Stau1 clusters are present only in dendritic shafts. Some of these clusters are transported along dendrites by microtubule-based molecular motors (KIF5). Stau1-negative clusters are preferentially translocated to dendritic spines by an actin-based molecular motor (myosin Va). This oriented translocation involves activation of the mGluR5 pathway.