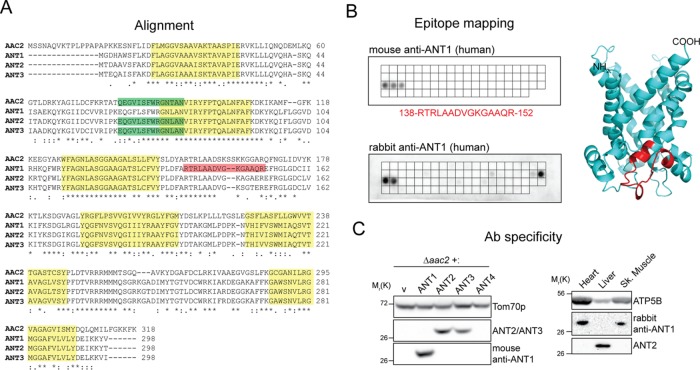
FIGURE 1:
Isoform-specific antibodies are capable of distinguishing the highly homologous ANT proteins. (A) Human adenine nucleotide translocases are highly conserved and share significant similarity with Aac2p, the major isoform in S. cerevisiae. Highlighted in red and green are epitopes recognized by ANT1 (mouse mAb) and ANT2/ANT3 mAbs, respectively; transmembrane helices are in yellow. (B) The epitope recognized by the newly generated ANT1-specific mAb was mapped using a peptide array (top) and found to be RTRLAADVGKGAAQR. The epitope is also highlighted in red in the structure of human ANT1 modeled using bovine ANT1 (Protein Data Bank 1OKC) as template. A peptide array (bottom) was also used to determine the epitopes recognized by the corresponding rabbit polyclonal ANT1. (C) Yeast extracts (left) derived from Δaac2 heterologously expressing yhANTs or empty vector were resolved by SDS–PAGE and immunoblotted with the ANT1-specific mAb or the ANT2/ANT3 mAb to test for isoform specificity. Tom70p, an outer mitochondrial membrane protein, serves as a loading control. Mitochondria (50 μg) from mouse liver, heart, and skeletal muscle (right) were similarly resolved to test the specificity of the rabbit ANT1 antiserum.