Figure 3.
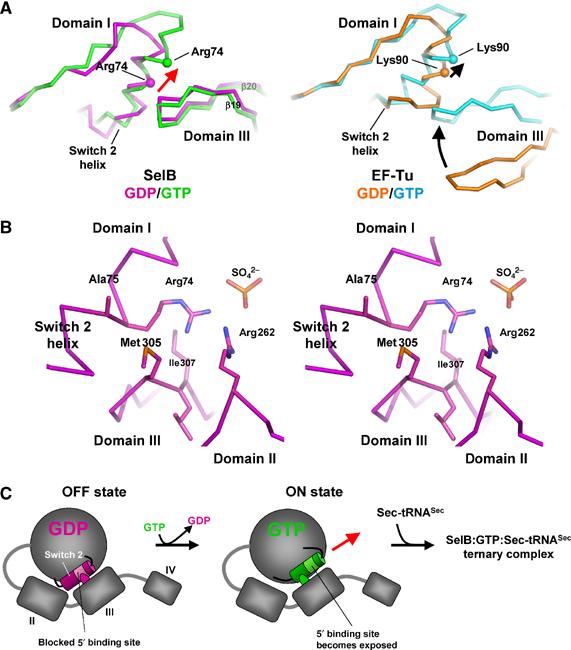
Conformational changes in the Switch 2 region of SelB domain I induced by GppNHp binding lead to the exposure of residues proposed to be involved in clamping the 5′ phosphate of the tRNA. (A) Comparison of the GDP and GTP conformations of SelB (molecule A, left) and EF-Tu (right). In SelB, nucleotide exchange leads to a conformational change restricted to the Switch 2 helix. Domains II, III and IV retain their relative positions, whereas the contact between domain III and Switch 2 is shifted by one helix turn (red arrow). EF-Tu, in contrast, undergoes large conformational changes that include, in addition to Switch 2, the movement of domain III towards the Switch 2 helix (black arrows). (B) Stereo view of the Switch 2 helix in contact with domains II and III from SelB:GDP (molecule C). Switch 2 residues Arg74 and Ala75 that may be involved in clamping the tRNA 5′ phosphate are blocked by several contacts with residues from domains II and III. (C) Model of the coupling between nucleotide and tRNA binding in SelB. In spite of adopting an overall ‘GTP-like' conformation, SelB:GDP binds tRNA with lower affinity because the 5′ phosphate binding site is blocked by domain III (left). Upon GTP binding, the movement of the Switch 2 helix by one helix turn leads to the exposure of the tRNA-binding site and the formation of a ternary complex.
