Figure 4.
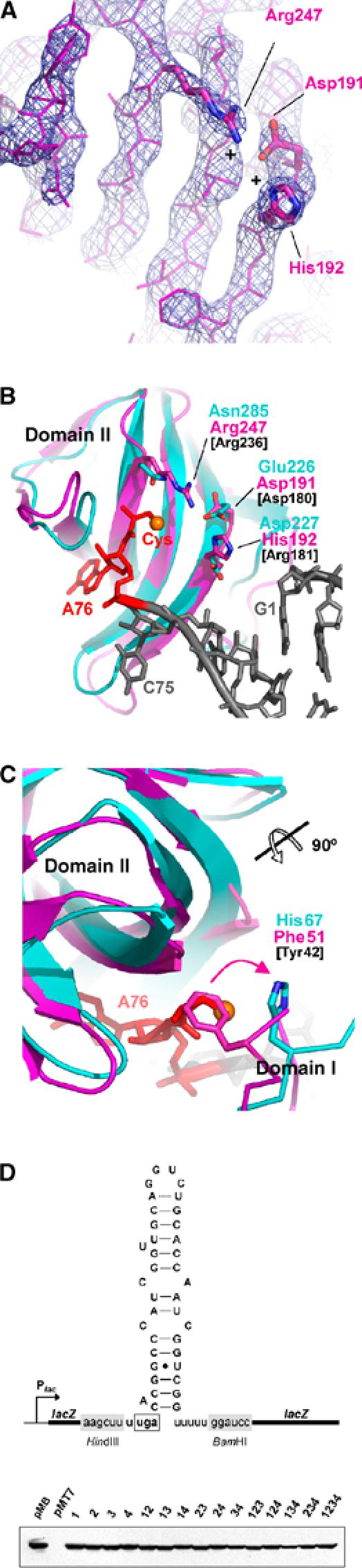
Aminoacyl-binding pocket of SelB and superposition with the corresponding EF-Tu:GppNHp:Cys-tRNACys region. M. maripaludis SelB is coloured in magenta, EF-Tu in cyan, the tRNA in grey and its terminal 3′Cys-A76 in red. Amino acids of E. coli SelB that in the mutational analysis were replaced by the corresponding EF-Tu residues are labelled in black. (A) 2Fo−Fc electron density map of the aminoacyl-binding pocket from SelB molecule C, contoured at 2.5 σ. Two positively charged residues (Arg247 and His192) may compensate for the negatively charged selenium. (B, C) Superposition of SelB and EF-Tu demonstrating the differences between key residues involved in aminoacyl binding. As a reference, the sulphur atom of the cysteyl moiety is displayed as an orange sphere. In SelB, Phe51 from domain I protrudes into the aminoacyl-binding pocket, thereby occupying the position of the modelled cysteyl side chain. (D) Mutational analysis of the aminoacyl-binding pocket from E. coli SelB. A scheme of the lacZ reporter gene carried by plasmid pWT is shown in the upper panel. The sequence of the UGA-SECIS cassette inserted into the 5′ portion of lacZ is depicted as mRNA sequence in its predicted secondary structure. The fusion gene is transcribed from the lac promoter (Plac). In the lower panel, an immunoblot analysis of cells expressing SelB variants with exchanges in the aminoacyl-binding pocket is shown as a control. Aliquots of cells used to determine the β-galactosidase activities listed in Table II were lysed and probed with anti-SelB antiserum. The numbering of the lanes relates to the numbering of the respective plasmids as given in Table II.
