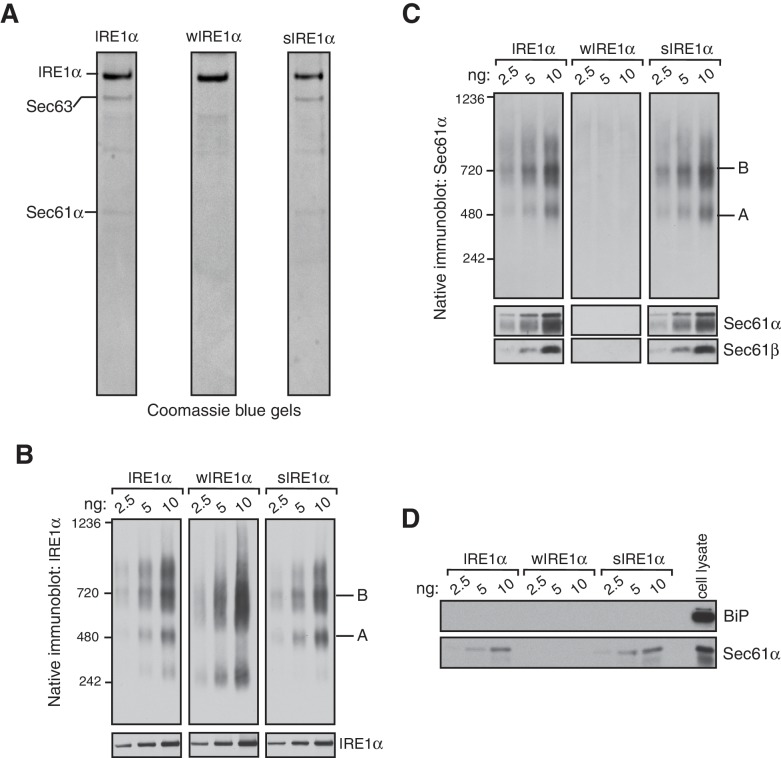
Figure 2. IRE1α forms a hetero-oligomeric complex with the Sec61 translocon.
(A) Coomassie blue stained gels showing IRE1α variants that were purified from HEK293 cells stably expressing 2X strep-tagged IRE1α. (B) The indicated concentration of purified IRE1α proteins was analyzed by BN-PAGE based immunoblotting with IRE1α antibodies. (C) The purified IRE1α proteins were analyzed as in panel B using Sec61α antibodies. (D) The purified IRE1α proteins were analyzed by standard immunoblotting with BiP and Sec61α antibodies.