Figure 2.
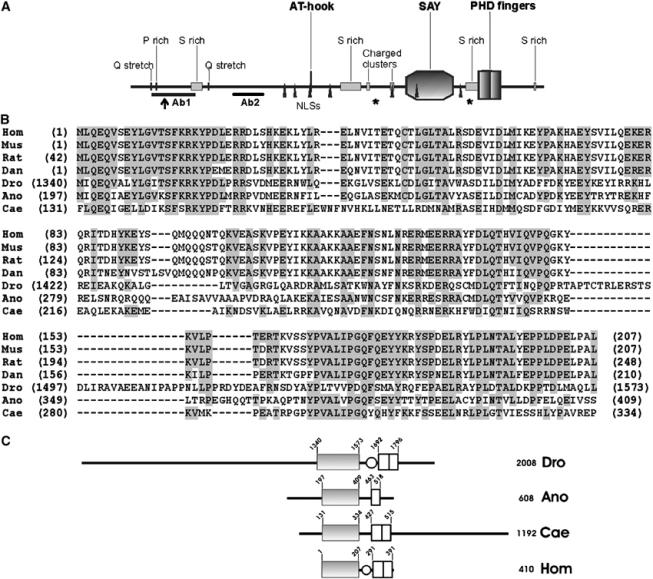
SAYP is a multidomain protein that has homologues in different species. (A) The structure of SAYP. AT-hook, SAY domain (amino-acid residues 1340–1573), PHD fingers (1692–1796), Ser-rich regions (293–345, 1018–1117, 1629–1693, 1960–1970), Pro-rich region (119–126), Gln stretches (93–97, 375–379), positively charged regions (1146–1160, 1297–1303), and nuclear localization signals (770–777, 805–811, 881–884, 991–997, 1297–1305, 1417–1420, 1610–1616) are indicated. The beginning of the short alternative form (residue 164) is indicated by an arrow. Asterisks mark the last amino acid of the protein in e(y)3u1 (1652) and e(y)3EMSl (1175) alleles. The positions of peptides used to raise antibodies are indicated (Ab1 and Ab2). (B) Sequence alignment of the SAY domains from proteins of different species. Hom, Homo sapiens, NP_060758 locus; Mus, M. musculus, NP_077212 locus; Rat, Rattus norvegicus, XP_214780 locus; Dan, D. rerio, AAH57492 locus; Dro, Drosophila melanogaster, SAYP; Ano, A. gambiae, XP_321190 locus; Cae, C. elegans, A88925 locus; amino acids identical to human are background-shaded when present in more than four species. (C) Comparison of SAYP domain structure with homologues from A. gambiae (Ano), C. elegans (Cae) and vertebrate (Hom). Solid box stands for the SAY domain, light box for the PHD finger, and oval for the Ser-rich region.
