Abstract
BACKGROUN/OBJECTIVES
Although studies have revealed that black garlic is a potent antioxidant, its antioxidant mechanism remains unclear. The objective of this study was to determine black garlic's antioxidant activities and possible antioxidant mechanisms related to nuclear factor erythroid 2-like factor 2 (Nrf2)-Keap1 complex.
METHODS/MATERIALS
After four weeks of feeding rats with a normal fat diet (NF), a high-fat diet (HF), a high-fat diet with 0.5% black garlic extract (HF+BGE 0.5), a high-fat diet with 1.0% black garlic extract (HF+BGE 1.0), or a high-fat diet with 1.5% black garlic extract (HF+BGE 1.5), plasma concentrations of glucose, insulin,homeostatic model assessment of insulin resistance (HOMA-IR) were determined. As oxidative stress indices, plasma concentrations of thiobarbituric acid reactive substances (TBARS) and 8-isoprostaglandin F2α (8-iso-PGF) were determined. To measure antioxidant capacities, plasma total antioxidant capacity (TAC) and activities of antioxidant enzymes in plasma and liver were determined. The mRNA expression levels of antioxidant related proteins such as Nrf2, NAD(P)H: quinone-oxidoreductase-1 (NQO1), heme oxygenase-1 (HO-1), glutathione reductase (GR), and glutathione S-transferase alpha 2 (GSTA2) were examined.
RESULTS
Plasma glucose level, plasma insulin level, and HOMA-IR in black garlic supplemented groups were significantly (P < 0.05) lower than those in the HF group without dose-dependent effect. Plasma TBARS concentration and TAC in the HF+BGE 1.5 group were significantly decreased compared to those of the HF group. The activities of catalase and glutathione peroxidase were significantly (P < 0.05) increased in the HF+BGE 1.0 and HF+BGE 1.5 groups compared to those of the HF group. The mRNA expression levels of hepatic Nrf2, NQO1, HO-1, and GSTA2 were significantly (P < 0.05) increased in the HF with BGE groups compared to those in the HF group.
CONCLUSIONS
The improvements of blood glucose homeostasis and antioxidant systems in rats fed with black garlic extract were related to mRNA expression levels of Nrf2 related genes.
Keywords: Garlic, antioxidant, oxidative stress, glucose, insulin
INTRODUCTION
Reactive oxygen species (ROS) are free radicals that accelerate prooxidant processes and produce toxicity in the body. Imbalance between prooxidant and antioxidant activities in human body can result in oxidative stress. Long term high-fat diet intake can enhance oxidative stress and subsequently increase the incidence of metabolic diseases such as diabetes, hyperinsulinemia, and cardiovascular diseases [1,2]. Hence, antioxidants are needed to scavenge these ROS and make them inactive. Garlics and processed garlics (black garlic) contain various antioxidant compounds such as flavonoids and organosulfur components that can prevent oxidative stress [3,4,5,6]. Garlic has been reported to have naturally occurring Hydrogen sulfur (H2S)-donating compounds [7]. H2S has been found to have many therapeutic potentials as an antioxidant for various diseases such as heart-related diseases, chronic inflammatory diseases, and Alzheimer's disease [8,9].
Black garlic is produced by aging fresh garlic at high temperature and high humidity [4,5]. During the aging process, polyphenol contents are increased and unstable compounds of raw garlic are converted into stable compounds such as S-allylcysteine (SAC) and S-allylmercaptocysteine (SAMC) [10,11]. These organosufur compounds are also considered as synthetic H2S donors as well as antioxidant. These compounds have been shown to be able to reduce the accumulation of ROS and appear to selectively induce nuclear factor erythroid 2-like factor (Nrf2) involved in oxidative stress defense [12].
Nrf2 proteins can bind to antioxidant response element (ARE). Nrf2 can also induce phase 2 target genes such as heme oxygenase-1(HO-1), superoxide dismutase (SOD), catalase (CAT), NAD(P)H: quinone-oxidoreductase-1 (NQO1), and glutathione S-transferase (GST) [13].
Although studies have revealed that black garlic is a potent antioxidant, its antioxidant mechanism remains unclear. Therefore, the objective of this study was to determine black garlic's antioxidant activities and possible antioxidant mechanisms related to Nrf2-Keap1 complex.
MATERIALS AND METHODS
Animals and Diet
Forty Sprague-Dawley male rats (4 weeks old) were preliminarily fed with a solid diet (Samyang Feed Co., Ltd.) for 3 days, and then classified according to body weight. Rats were first divided into two groups and fed normal diet (NF group, n = 8) and high-fat diet (HF group, n = 32) for one week. After that, rats fed with HF diet were further divided into 4 groups with experimental diets; a high-fat diet (HF, n = 8), a high-fat diet with 0.5% black garlic extract (HF+BGE 0.5, n = 8), a high-fat diet with 1.0% black garlic extract (HF+BGE 1.0, n = 8), or a high-fat diet with 1.5% black garlic extract (HF+BGE 1.5, n = 8). Rats were fed with experimental diets for another 4 weeks.
The NF group was fed with standard AIN-93G diet containing 200 g of casein, 70 g of soybean oil (7% fat), and 730 g of carbohydrate per kg diet [14]. The HF group was fed with 200 g of fat (130 g of lard and 70 g of soy bean oil, 20% fat) and 200 g of casein per kg diet. The amount of carbohydrate in HF groups was adjusted corresponding to the amount of black garlic as a dietary supplement: 490 g/kg for HF group (no black garlic supplement), 485 g/kg for HF+BGE 0.5 (0.5% black garlic supplement), 480 g/kg for HF+BGE 1.0 (1.0% black garlic), and 475 g/kg for HF+BGE 1.5 (1.5% black garlic). The vitamin and mineral contents of HF diet was the same as NF diets including 0.035% mineral mixture and 0.01% vitamin mixture. Aged black garlic extract (60% Brix concentrate) was provided by Korea Euisung Black Garlic Agricultural Association Corporation (Euisung, Korea).
Each animal was placed in a stainless steel cage in which water was freely provided. The temperature of the cage was maintained at 24 ± 1℃. All experimental diets were refrigerated at 4℃ during the experimental period. Dietary intake was measured twice a week, and body weight was measured once a week at regular intervals to calculate dietary efficiency. All animal experiments were carried out in accordance with the Dankook University Animal Experimental Ethics Committee Guidelines (approval no: 12-004).
Blood and liver sample preparation for analysis
After 4 weeks of feeding, rats were fasted for 12 h before sacrifice, and then anesthetized with ethyl ether. Blood was collected from the heart using a 10 mL syringe. The collected blood was placed in a sodium heparin-coated tube and centrifuged at 3,000 rpm (4℃) for 15 minutes (Beckman J2-21, USA) to obtain plasma. After obtaining blood, rat livers were removed, washed with 0.9% NaCl solution, and then dehydrated with filter paper. Liver and plasma sample were stored in a -70℃ refrigerator for further analysis.
Plasma glucose and insulin concentrations and insulin sensitivity
The changes in plasma glucose and insulin concentrations were analyzed after the end of the administration of black garlic. Plasma glucose concentration was measured by using a glucose assay kit (Asan Pharmaceutical Co., Seoul, Korea). Plasma insulin concentration was measured by ELIZA method (Shibayagi Co., Shibukawa, Japan). Homeostatic model assessment insulin resistance (HOMA-IR) is a marker of insulin sensitivity, and it was calculated using fasting plasma insulin (FPI) and glucose (FPG) concentrations according to the following formula: HOMA-IR = [FPG (mg/dL) * FPI (uU/mL)]/2430, where 2430 is a constant derived from assuming that HOMA-IR of young adult rats is an average of 1 [15,16]. Fasting glucose to insulin ratio (FGIR) was the ratio of FPG divided by FPI levels (FPG/FPI). To convert glucose between mmol/L and mg/dL, the following equation was used: 1 mmol/L = 18 mg/dL [16].
The level of plasma lipid oxidation assay
The level of plasma lipid oxidation was measured by the concentration of malondialdehyde (MDA), which is produced during blood lipid oxidation [17]. After adding 8.1% (w/v) sodium dodecyl sulphate, 20% (v/v) acetic acid (pH 3.5), and 0.8% Thiobarbituric acid (TBA) in 100 µL of plasma, distilled water was added to make a total volume of 2 mL. After being incubated in a water bath at 95℃ for 30 min, cold distilled water, and the mixture of butanol and pyridine was added afterwards. After vortexing, the mixture was centrifuged at 800 rpm for 10 minutes and absorbance was measured at 532 nm. The MDA standard curve was prepared by the various concentrations of 1,1,3,3-tetraethoxy propane (TEP). After subtracting the blank value from the sample value, the concentration is calculated by substituting the value into the standard curve, and the concentration was expressed as TBARS concentration (nmol/mL).
The concentration of plasma and 8-isoprostaglandin F2α (8-iso-PGF) level was determined by using an enzyme immunoassay kit (Assay Designs Inc. Ann Arbor, MI). The color change of each sample was measured for optical density at 450 nm in an ELISA reader, and the concentration of plasma 8-iso-PGF in the samples was calculated by using the optical density of standard curve.
Total antioxidant capacity (TAC) assay
TAC in plasma was measured by modified Trolox equivalent antioxidant capacity (TEAC) method [18]. At acidic pH, colorless reduced 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonate, ABTS) was oxidized to cyan ABTS+ by H2O2. When antioxidants were present in the extract, ABTS+ was discolored proportionally to these concentrations and the results of this color change reaction were investigated with absorbance at 660 nm. For the TAC measurement of the sample extracts, standard curves were prepared using 0, 2.25, 4.5, 9.0, 22.5, 33.75 and 45 nmol of Trolox as standard reagents. TAC activity in plasma was expressed as nmol Trolox equivalent.
Activities of antioxidant enzymes
Antioxidant enzyme activities of catalase, SOD, and GSH-Px were determined both in plasma and liver. To measure enzyme activities in liver of rats, liver (0.2 g) was chopped and homogenized with a buffer solution containing 0.1 M triethanolamine, 0.02 M EDTA (ethylenediamine tetracetate, pH 7,4) and 0.002 M DTT (dithiothreitol). The homogenized sample was centrifuged at 600 × g (4℃) for 10 minutes, and then the supernatant was removed and re-centrifuged at 10,000 × g (4℃.) for 20 minutes [19,20]. The activity of catalase, an enzyme that decomposes H2O2 into water and oxygen, was measured briefly by modifying Aebi's study [21]. Sample was added to 50 mM potassium phosphate buffer (pH 7.4), reacted at 25℃ for 5 minutes, and then added with 340 mM H2O2 solution at 25℃ for 5 minutes. The absorbance change was measured. Enzyme activity (one catalase unit) was calculated as the amount of enzyme required to decompose 1 mM of H2O2/min. SOD activity was measured with a SOD assay kit (Sigma, MO, USA) [22]. In this assay, xanthine-xanthine oxidase system was used to generate free radicals. The degree of removal of free radicals by the antioxidant enzyme, SOD, was measured at 440 nm in terms of color development. The SOD activity (inhibition rate) was determined using standard curves of various concentrations of SOD. The activity of GSH-Px was measured by modifying the experimental method of Paglia and Valentine [23]. Reduced glutathione (GSH) was oxidized by GSH-Px to glutathione (GSSG), and the absorbance change at 340 nm when the GSSG is reduced by nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), was measured. One GSH-Px unit was expressed as the amount of NADPH oxidized by 1 g of sample per minute. Liver protein level was measured using the method of Lowry et al. [24]. Plasma and liver activities of enzyme were expressed in unit per milliliter plasma (U/mL) and unit per milligram tissue protein (U/mg protein), respectively.
mRNA expression of proteins related with antioxidant mechanisms in liver tissue
In order to confirm the antioxidant mechanism of black garlic extract, mRNA in black garlic extract was isolated using Trizol reagent (Sigma, MO, USA) and cDNA was synthesized using reverse transcriptase (RT, Promega Corp., WI, U.S.A) from mRNA [25]. One mL of Trizol was added to finely chopped liver tissue (0.2 g) and allowed to vortex for 5 minutes. Subsequently, 200 µL of chloroform (Sigma-Aldrich, MO, USA) was added to the mixture, vortexed for 5 minutes, and centrifuged at 10,500 rpm for 15 minutes to collect supernatant. Isopropanol was added to the supernatant, vortexed for 3 minutes, allowed to stand at room temperature for 5 to 10 minutes, centrifuged at 10,500 rpm for 10 minutes at 4℃, after which the supernatant was removed. 50 µL of RNase-free dH2O / 0.1 mM EDTA was added to the dried pellet to determine the concentration of RNA in pellet sample. The purity (1.7-2.0) of the RNA was determined at the OD260 / OD280 ratio, and the RNA concentration was calculated at the OD260 value. The cDNA from the extracted RNA was synthesized by using 3 µg of isolated total RNA with 0.5 µg Oligo DT, reaction mixture buffer (4 µL of 5 × first standard buffer, 2 µL of 0.1 M DTT, 1 µL of 10 mM NTP), and SuperScript Ⅱ reverse transcriptase (Invitrogen, CA, USA).
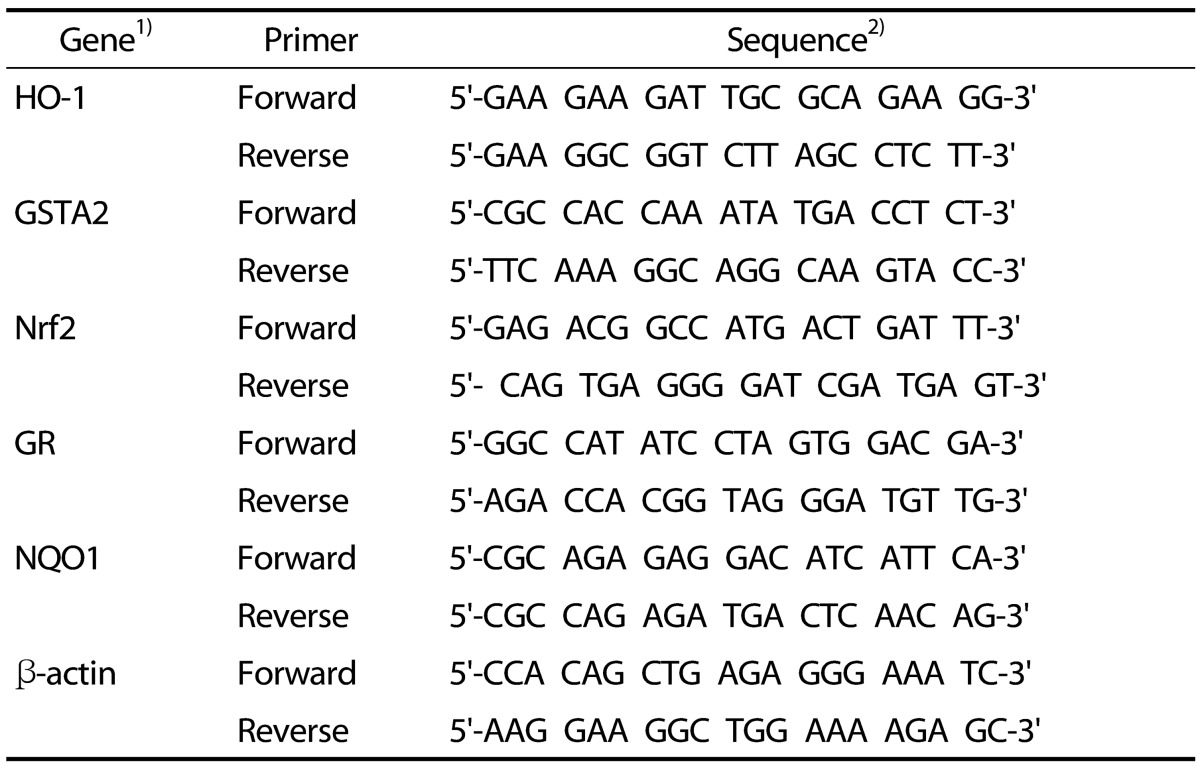
The mRNA expression levels of transcription factor, Nrf2, HO-1, NQO-1, GR, and GSTA2, as antioxidant markers were compared with the mRNA expression of β-actin as loading control. Real-time RT-PCR was performed using Applied Biosystems Step One (Applied Biosystems, CA, USA) software v2.1). cDNA template (2 µL), SYBR Green Master Mix (Applied Biosystems, CA, USA), sense and Anti-sense primers (1 µL) were added to the wells of the system, and then the total volume of the well was adjusted to 20 µL by adding distilled water. The PCR conditions were pre-denaturation at 95℃ for 10 min, denaturation at 95℃ for 15 min, annealing at 60℃ for 1 min, extension at 95℃ for 15 min for 40 cycles. The result is calculated as the relative expression level of the expression level of beta-actin. Table 1 shows the specific SYBR gene expression assays used in the experiments.
Table 1. Primer sequence related to antioxidants.
1)HO-1, heme oxygenase-1; GSTA2, glutathione S-transferase alpha 2; Nrf2, nuclear factor erythroid 2-like factor 2; GR, glutathione reductase; NQO1, NAD(P)H: quinone-oxidoreductase-1.
2)T, Thymine; A, Adenine; C, Cytosine; G, Guanine
Statistical analysis
Statistical analysis was performed using the Statistical Analysis System software 9.4 (SAS Institute, Cary, NC, USA). To compare the mean values among the five groups, one-way analysis of variance (ANOVA) was used. The Duncan's multiple range test was used for the post test. Statistical significance was defined as a P-value less than 0.05
RESULTS
Mean weekly weight changes
The weekly weight changes of the HF+BGE 1.5 group, not the HFBGE 0.5 group or the HF+BGE 1.0 group, were significantly (P < 0.05) lower than those of the HF group (Table 2). No significant difference in weekly food intake change or FER among groups was observed.
Table 2. Weekly changes of weight, food intake, and food efficiency ratio of experimental diets for 5 weeks.
1)NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE
2)Mean ± SD
3)The significant was determined by Duncan's multiple range test at α = 0.05
4)FER (food efficiency ratio) = Body weight gain for experimental period / Food intake for experimental period
Plasma glucose, insulin, and HOMA-IR levels
Plasma glucose concentrations in HF+BGE 0.5, HF+BGE 1.0, and HF+BGE 1.5 groups were significantly (P < 0.05) lower those in the HF group (Table 3). However, there was no dose-dependent significance among HF+BGE groups. Plasma insulin concentration was significantly (P < 0.05) lower in the HF+BGE 1.0 group and the HF+BGE 1.5 group, but not the HF+BGE 0.5 group compared to that in the HF group. As indices of insulin sensitivity, both HOMA-IR level and FGIR were significantly (P < 0.05) lower in the black garlic extract supplemented groups (HF+BGE 0.5, HF+BGE 1.0, and HF+BGE 1.5) than those in the HF group. However, there was no dose-dependent effect among black garlic supplemented groups.
Table 3. The levels of glucose, insulin, and homeostasis model assessment of insulin of experimental diets.
1)NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE.
2)Mean ± SD
3)The significant was determined by Duncan's multiple range test at α = 0.05
4)HOMA-IR, homeostasis model assessment of insulin resistance
5)FGIR, fasting glucose to insulin ratio
Plasma levels of TBARS concentration and 8-iso-PGF
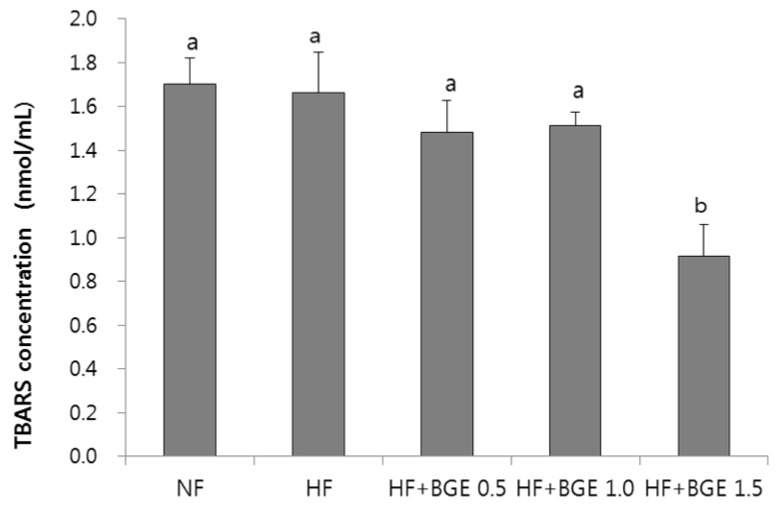
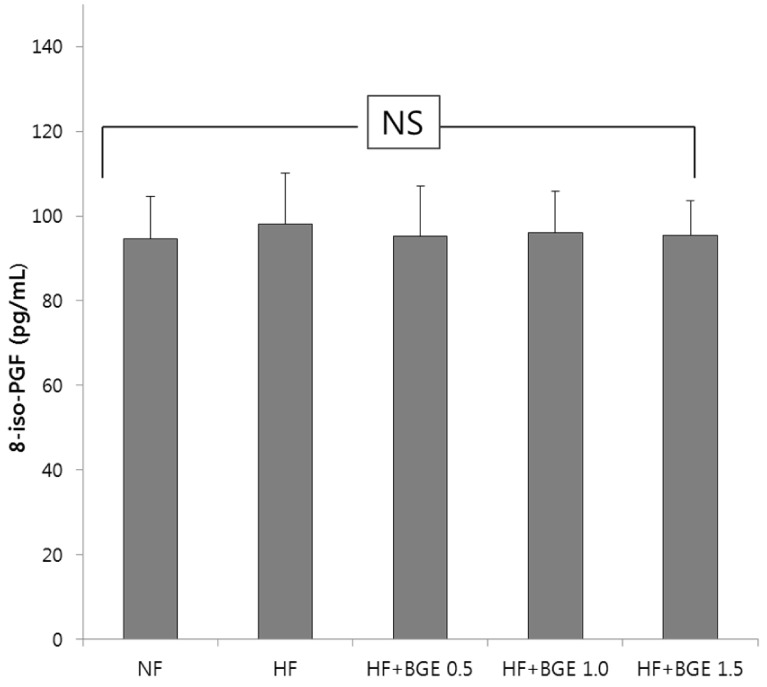
Plasma TBARS concentration in the HF+BGE 1.5 group was significantly (P < 0.05) decreased compared to that of the HF group (Fig. 1). However, no significant change in plasma TBARS concentration was found among HF, HF+BGE 0.5, and HF+BGE 1.0 groups. Plasma level of 8-iso-PGF was not significantly different among all groups (Fig. 2).
Fig. 1. TBARS concentration s after the supplementation of black garlic extracts.
Rats were fed with 5 different diets for 5 weeks; NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE. Each bar represents the mean ± SD. Different letters above each bar indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Fig. 2. 8-iso-PGF2 changes after the supplementation of black garlic extracts.
Rats were fed with 5 different diets for 5 weeks; NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE. Each bar represents the mean ± SD. NS above the bars indicate no significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Plasma total antioxidant capacity
Plasma TAC concentration in the HF group was significantly (P < 0.05) lower than that in the NF group (Fig. 3). Plasma TAC concentrations in the HF+BGE 1.0 and HF+BGE 1.5 groups were significantly (P < 0.05) increased compared to that of in the HF group.
Fig. 3. Total antioxidant capacity changes after the supplementation of black garlic extracts.
Rats were fed with 5 different diets for 5 weeks; NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE. Each bar represents the mean ± SD. Different letters above each bar indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Activities of antioxidant enzymes
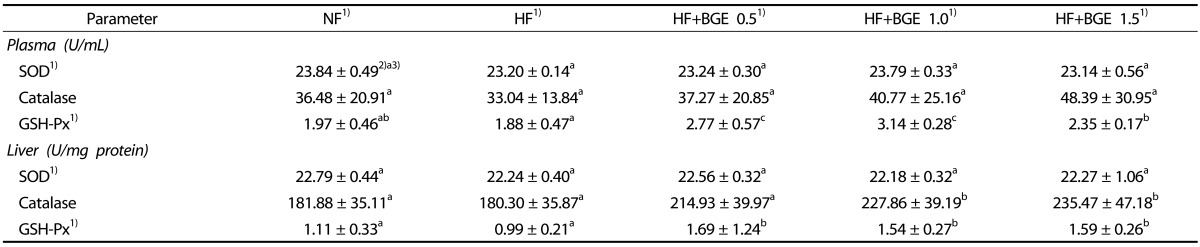
No significant differences in plasma SOD and catalase activities were found among groups (Table 4). Plasma GSH-Px concentrations in HF+BGE 1.0 and HF+BGE 1.5 groups were significantly (P < 0.05) higher than those in the HF group. In liver antioxidant enzymes, no significant difference in SOD activity was observed among groups. However, the activities of catalase and GSH-Px in HF+BGE 1.0 and HF+BGE 1.5 groups were significantly (P < 0.05) increased compared to those in the HF group.
Table 4. Antioxidant enzyme activities in the plasma and liver of rats fed experimental diets.
1)NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
2)Mean ± SD
3)The significant was determined by Duncan's multiple range test at α = 0.05
mRNA expression levels of transcription factors and enzymes
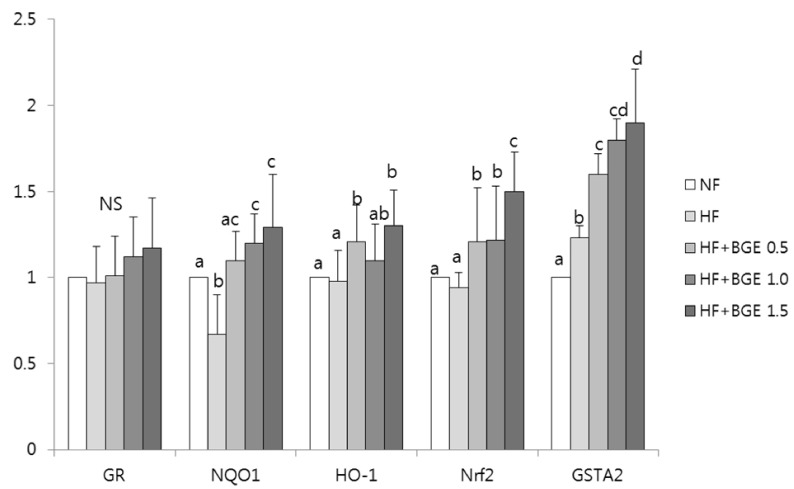
The mRNA expression levels of GR were not significantly different among groups (Fig. 4). The mRNA expression levels of Nrf2 in black garlic extract groups (HF+BGE 0.5, HF+BGE 1.0, and HF+BGE 1.5) were significantly (P < 0.05) higher than those in the HF group. However, there was no dose-dependent effect of black garlic on the mRNA expression of Nrf2. The mRNA expression levels of hepatic NQO1, HO-1, and GSTA2 were significantly (P < 0.05) increased after treatments with black garlic extract compared to those in the HF group. However, there was no dose-dependent effect of black garlic on these mRNA expression levels.
Fig. 4. Effect of black garlic extract on mRNA expression of transcription factor and antioxidants in liver of rats.
Rats were fed with 5 different diets for 5 weeks; NF, Normal fat (7% fat); HF, High-fat (20% fat + 1% cholesterol); HF+BGE 0.5, High-fat + 0.5% black garlic extract (BGE); HF+BGE 1.0, High-fat + 1.0% BGE; HF+BGE 1.5, High-fat + 1.5% BGE. Total RNA was isolated using TRI-reagent, and cDNA was synthesized using 3 µg of total RNA with SuperScript II reverse transcriptase. Real-time PCR was performed using SYBR green and standard procedures to assess the mRNA expression of the primer in liver samples obtained from each group. An Applied Biosystems StepOne softwere v2.1 was used. Each bar represents the mean ± SD of three independent experiments. Different letters above each bar indicate significant differences among the groups at α = 0.05 as determined by Duncan's multiple range tests. GR, glutathione reductase; NQO1, NAD(P)H:quinone-oxidoreductase-1; HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-like factor; GSTA2, glutathione S-transferase alpha 2.
DISCUSSION
In the present study, 1.5% black garlic extract treatments resulted in decreased body weight. No significant changes in food consumption were detected in any of the black garlic treated groups, suggesting that the weight loss was not associated with decreased food consumption. These results are in agreement with previous studies of Shin et al. [27] showing that aged black garlic administration can lead to decreased epididymal and total fat pad and liver weights of rats. Jung et al. [28] have also reported that black garlic has favorable weight reduction effects in rats fed with high-fat. Based on these results, it can be expected that aged black garlic extract can help reduce weight without affecting food intake.
Hyperinsulinemic euglycemic glucose clamp test is the most accurate method for determining insulin resistance [16]. However, this method is inconvenient and too complicated to be used in clinical settings. Thus, HOMA-IR is often used clinically as an alternative method for the determination of fasting insulin level because the results from HOMA-IR method are quite similar to the results from hyperinsulinemic euglycemic glucose clamp [17]. Black garlic administration in high-fat diet improved plasma insulin and glucose level as well as homeostatic model assessment insulin resistance. These results are consistent with the results of Seo et al. [30]'s study, which shown that black garlic consumption increased serum insulin levels by 12.1% and decreased HOMA-IR value. The anti-diabetic effect of black garlic is known to be caused by substances called organosufur compounds, which is produced during processing of black garlic, to stimulate beta-cells to increase insulin secretion [31,32]. Thus, our data could have suggested that aged black garlic supplemented at the level of up to 1.5% of diet significantly improved blood glucose homeostasis, possibly by increasing plasma insulin level or alleviating insulin resistance. Especially insulin resistance is an important factor for causes of type 2 diabetes.
Increased lipid peroxidation and depleted antioxidant system have been reported as oxidative stress markers. This study demonstrated that total antioxidant capacity and lipid peroxidation were improved significantly in the HF+BGE 1.5 group than those in the HF group, suggesting that high levels of consumption of black garlic extracts could reduce oxidative stress in mice with high-fat diet. In addition, the activities of plasma or liver GSH-Px and liver catalase in all HF+BGE groups were significantly (P < 0.05) increased compared to those in the HF group.
Previous studies have demonstrated that aged black garlic has strong anti-oxidant activity [4,5,6,27,28,32,33,34]. In vitro studies have reported that the free radical scavenging ability of aged black garlic is 4.5 times higher than fresh garlic, demonstrating that ageing of whole garlic can enhance antioxidant activity [4,33]. Another study has also shown that aged black garlic has 3 to 9-fold stronger SOD activities than fresh garlic [5]. The reason why black garlic has more antioxidant activity than fresh garlic is that a large amount of organosulfur compounds are produced during the aging process of garlic [10]. Thiol group in organosulfur compounds of black garlic can easily donate proton to an electrophilic species, thereby neutralizing them or making them less reactive [32,34].
The concentration of 8-iso-PGF is often measured as an indicator of oxidative stress. Compound 8-iso-PGF is formed by a non-enzymatic attack by ROS on arachidonic acid of cell membranes. In the present study, the concentration of 8-iso-PGF in plasma after supplementation of high-fat and black garlic extract was measured. However, there was no statistical significance among groups. This is probably because blood, especially plasma, is not a good sample for measuring 8-iso-PGF level, although we used an assay kit specific for blood sample. It has been reported that urine sample is more preferable than blood sample for measuring 8-iso-PGF level because of the non-invasiveness of the procedure and the lack of ex vivo artefactual formation resulting from auto-oxidation of lipids [35]. Another reason is that oxidative stress caused by high-fat diet is not high enough to measure the changes of plasma 8-iso-PGF level.
In addition to demonstrating the potential of black garlic extract in improving blood glucose control as well as decreasing oxidative stress, we also sought the mechanism by which Nrf2 activation by black garlic extract is able to provide those beneficial effects.
When we determined the mRNA expression levels of antioxidant related proteins, the mRNA expression levels of hepatic Nrf2 and GSTA2, HO-1, and NQO1 in HF+BGE 1.5 group were significantly (P < 0.05) increased compared to those of the HF group, indicating induction of Nrf2 pathway by black garlic extract. Nrf2 has been recognized as a major transcription factor in protecting cells against oxidative stress. It can activate the transcription of antioxidant enzymes such as HO-1, gluthethione reductase (GR), SOD, CAT, NQO1, and GSTA2 [13].
In this study, activation of Nrf2 pathway by black garlic extract has been shown to be effective in two aspects: regulation of hyperglycemia and reduction of oxidative damage caused by high fat intake. Several studies have focused on the therapeutic potential of Nrf2 activation in diabetes [36,37,38], implicating control of oxidative stress in addition to regulation of inflammatory cytokines as methods of Nrf2 protection. Nrf2-knockout (KO) mice were not able to improve their resistance to insulin because of the lack of activation of the Nrf2/Keap1 system [36]. SAC treatment, one of major black garlic's components, in streptozotocin-diabetic rats lowered hippocampal inflammatory cytokines levels and prevent the reduction of Nrf2 and HO-1 [39].
Another benefit of black garlic treatment in this study was to improve antioxidant systems by activating Nrf2 pathway. Several compounds of garlics have been shown to be able to increase the mRNA expression levels of antioxidant enzymes such as HO-1, NQO1, and GSTs through activation of Nrf2 [39,40,41,42,43,44]. The major components of garlic and study models applied in such studies include allicin in hippocampus of Alzheimer's disease (AD) rat model [39] or hippocampus of cognitively impaired aged mouse [40] and organosulfur compounds such as diallyl sulfide (DAS), diallyl disulfide (DADS), dipropyl sulfide (DPS), and dipropyl disulfide (DPDS) in hepatic cytosols and microsomes of rats [41] or in carbon tetrachloride (CCl4)-induced oxidative hepatic damage in rat liver [42]. Those studies have suggested that garlic administration can decrease Keap1 protein levels, allowing the dissociation of the Keap1/ Nrf2 complex and the translocation of Nrf2 to the nucleus. In the nucleus, Nrf2 undergoes acetylation and binds to the enhancer regions called “antioxidant response element” (ARE) in the promoter region of cytoprotective genes, thus defending cells against free radical induced damage [20,33].
Although no study has reported how black garlic itself effects the mRNA expression of Nrf2 and its related antioxidant enzymes, several studies have shown that treatment with SAC, in aged black garlic, can result in an increase in Nrf2 protein level and subsequent activation of antioxidant enzymes [12,43,44]. When the antioxidative effects of SAC are investigated in vitro and in vivo model of cerebral ischemia, it has been shown that SAC can strongly induce Nrf2 target genes such as HO-1 [12]. In a study of rat striatum for protecting 6-hydroxydopamine-induced neurotoxicity, it has been shown that SAC can promote the activation of Nrf2 and the up-regulation of Nrf2-dependent genes encoding antioxidant enzymes HO-1, NQO-1, GR, and SOD [44].
Although we did not measure the types or concentration of organosulfur compounds in black garlic and this is a limitation of this study, it can be suggested, based on the above studies, that the antioxidative effect of black garlic is related to the up-regulation of Nrf2 dependent antioxidant enzymes. Future studies are needed to determine the antioxidative effects of black garlic depending on SAC content and the Nrf2 pathway to clarify the antioxidant mechanisms of black garlic.
In conclusion, black garlic treatment significantly attenuated hyperglycemia, decreased lipid peroxidation, and improved antioxidant systems, mediating through activating mRNA expressions of Nrf2 and Nrf2 downstream targets, such as NQO1, HO-1, and GSTA2.
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee OH, Kwon YI, Hong HD, Park CS, Lee BY, Kim YC. Production of reactive oxygen species and changes in antioxidant enzyme activities during differentiation of 3T3-L1 adipocyte. J Korean Soc Appl Biol Chem. 2009;52:70–75. [Google Scholar]
- 3.Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- 4.Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009;3:156–161. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MH, Kim MJ, Lee JH, Han JI, Kim JH, Sok DE, Kim MR. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J Med Food. 2011;14:732–738. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 6.Jang EK, Seo JH, Lee SP. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J Food Sci Technol. 2008;40:443–448. [Google Scholar]
- 7.Wang Q, Wang XL, Liu HR, Rose P, Zhu YZ. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H(2)S production. Antioxid Redox Signal. 2010;12:1155–1165. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 8.Bełtowski J. Hydrogen sulfide in pharmacology and medicine--an update. Pharmacol Rep. 2015;67:647–658. doi: 10.1016/j.pharep.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Bhuiyan AI, Papajani VT, Paci M, Melino S. Glutathione-garlic sulfur conjugates: slow hydrogen sulfide releasing agents for therapeutic applications. Molecules. 2015;20:1731–1750. doi: 10.3390/molecules20011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136:716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 11.Toledano-Medina MA, Pérez-Aparicio J, Moreno-Rojas R, Merinas-Amo T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016;199:135–139. doi: 10.1016/j.foodchem.2015.11.128. [DOI] [PubMed] [Google Scholar]
- 12.Shi H, Jing X, Wei X, Perez RG, Ren M, Zhang X, Lou H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J Neurochem. 2015;133:298–308. doi: 10.1111/jnc.12986. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Osorio AS, González-Reyes S, Pedraza-Chaverri J. Natural Nrf2 activators in diabetes. Clin Chim Acta. 2015;448:182–192. doi: 10.1016/j.cca.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Ha AW, Ying T, Kim WK. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr Res Pract. 2015;9:30–36. doi: 10.4162/nrp.2015.9.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Santos MT, Valles J, Aznar J, Vilches J. Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J Clin Pathol. 1980;33:973–976. doi: 10.1136/jcp.33.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice-Evans CA. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic Res. 2000;33(Suppl):S59–S66. [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Ha AW, Na SJ, Kim WK. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutr Res Pract. 2013;7:475–480. doi: 10.4162/nrp.2013.7.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 23.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Wang YP, Cheng ML, Zhang BF, Mu M, Wu J. Effects of blueberry on hepatic fibrosis and transcription factor Nrf2 in rats. World J Gastroenterol. 2010;16:2657–2663. doi: 10.3748/wjg.v16.i21.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin JH, Lee CW, Oh SJ, Yun J, Kang MR, Han SB, Park H, Jung JC, Chung YH, Kang JS. Hepatoprotective effect of aged black garlic extract in rodents. Toxicol Res. 2014;30:49–54. doi: 10.5487/TR.2014.30.1.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung YM, Lee SH, Lee DS, You MJ, Chung IK, Cheon WH, Kwon YS, Lee YJ, Ku SK. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr Res. 2011;31:387–396. doi: 10.1016/j.nutres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo YJ, Gweon OC, Im J, Lee YM, Kang MJ, Kim JI. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. J Food Sci Nutr. 2009;14:1–7. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Jeong YY, Ryu JH, Shin JH, Kang MJ, Kang JR, Han J, Kang D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules. 2016;21:430. doi: 10.3390/molecules21040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae SE, Cho SY, Won YD, Lee SH, Park HJ. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. Lebenson Wiss Technol. 2014;55:397–402. [Google Scholar]
- 34.Yang CS, Chhabra SK, Hong JY, Smith TJ. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr. 2001;131:1041S–1045S. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- 35.Patrono C, Falco A, Davì G. Isoprostane formation and inhibition in atherothrombosis. Curr Opin Pharmacol. 2005;5:198–203. doi: 10.1016/j.coph.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013;33:2996–3010. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiménez-Osorio AS, Picazo A, González-Reyes S, Barrera-Oviedo D, Rodríguez-Arellano ME, Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci. 2014;15:20290–20305. doi: 10.3390/ijms151120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Ghasemi Z, Roghani M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur J Pharmacol. 2017;794:69–76. doi: 10.1016/j.ejphar.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 40.Zhu YF, Li XH, Yuan ZP, Li CY, Tian RB, Jia W, Xiao ZP. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur J Pharmacol. 2015;762:239–246. doi: 10.1016/j.ejphar.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Li XH, Li CY, Xiang ZG, Hu JJ, Lu JM, Tian RB, Jia W. Allicin ameliorates cardiac hypertrophy and fibrosis through enhancing of Nrf2 antioxidant signaling pathways. Cardiovasc Drugs Ther. 2012;26:457–465. doi: 10.1007/s10557-012-6415-z. [DOI] [PubMed] [Google Scholar]
- 42.Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Antimutagenic activity of organosulfur compounds from Allium is associated with phase II enzyme induction. Mutat Res. 2001;495:135–145. doi: 10.1016/s1383-5718(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 43.Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174–185. doi: 10.1016/j.fct.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Tobón-Velasco JC, Vázquez-Victorio G, Macías-Silva M, Cuevas E, Ali SF, Maldonado PD, González-Trujano ME, Cuadrado A, Pedraza-Chaverrí J, Santamaría A. S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radic Biol Med. 2012;53:1024–1040. doi: 10.1016/j.freeradbiomed.2012.06.040. [DOI] [PubMed] [Google Scholar]