Figure 1.
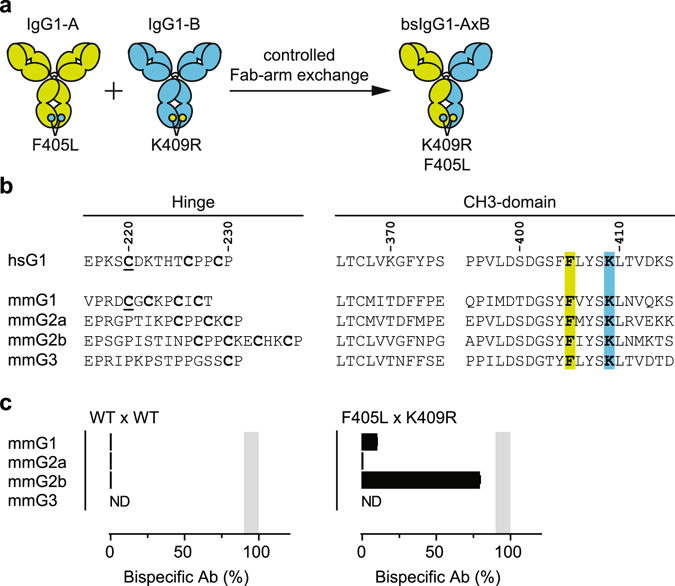
(a) Principle of controlled Fab-arm exchange (cFAE) for the generation of human IgG1-based bispecific antibodies. IgG1-A and IgG1-B molecules containing matching point mutations in their CH3 domains, F405L and K409R (EU numbering)23 respectively, are separately expressed and mixed in a 1 to 1 ratio under mild reducing conditions (5 h at 31 °C using 75 mM 2-MEA). This allows in the recombination of half-molecules driven by the matched mutations that enable dissociation of half-molecules in IgG1-A and IgG1-B and favor heterodimerization into IgG1-AB molecules. (b) Amino acid sequence alignment of human (H. sapiens; hs) and mouse (M. musculus; mm) hinge and CH3 regions. EU-numbering convention is used to annotate the amino acid residues. Cysteine residues in the hinge region involved in HC-HC linkage and LC-HC pairing (underlined) are indicated in bold. Residues F405 and K409 are indicated in green and blue, respectively. (c) Efficiency of cFAE as measured by HIC of mixtures of 2F8-derived and 7D8-derived mmIgG1, mmIgG2a or mmIgG2b parental antibodies, containing the indicated mutations (above panels). Data represent mean ± SEM of at least two separate experiments. ND = not done (due to limited sample availability). Shaded area represents 90–100% efficiency.
