Abstract
Background and Objectives
The burden of heart failure has increased in Korea. This registry aims to evaluate demographics, clinical characteristics, management, and long-term outcomes in patients hospitalized for acute heart failure (AHF).
Subjects and Methods
We prospectively enrolled a total of 5625 consecutive subjects hospitalized for AHF in one of 10 tertiary university hospitals from March 2011 to February 2014. Descriptive statistics were used to determine the baseline characteristics of the study population and to compare them with those from other registries.
Results
The mean age was 68.5±14.5 years, 53.2% were male, and 52.2% had de novo heart failure. The mean systolic and diastolic blood pressures were 131.2±30.3 mmHg and 78.6±18.8 mmHg at admission, respectively. The left ventricular ejection fraction was ≤40% in 60.5% of patients. Ischemia was the most frequent etiology (37.6%) and aggravating factor (26.3%). Angiotensin converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, and aldosterone antagonists were prescribed in 68.8%, 52.2%, and 46.6% of the patients at discharge, respectively. Compared with the previous registry performed in Korea a decade ago, extracorporeal membrane oxygenation (ECMO) and heart transplantation have been performed more frequently (ECMO 0.8% vs. 2.8%, heart transplantation 0.3% vs. 1.2%), and in-hospital mortality decreased from 7.6% to 4.8%. However, the total cost of hospital care increased by 40%, and one-year follow-up mortality remained high.
Conclusion
While the quality of acute clinical care and AHF-related outcomes have improved over the last decade, the long-term prognosis of heart failure is still poor in Korea. Therefore, additional research is needed to improve long-term outcomes and implement cost-effective care.
Keywords: Heart failure, acute heart failure; Mortality; Guideline adherence; Quality of health care; Treatment outcome
Introduction
Heart failure (HF) is a major global health problem, with a prevalence of more than 26 million annual cases worldwide.1),2) The prevalence is increasing in many countries due to aging societies, increased prevalence of risk factors, and better survival from other cardiovascular diseases.3),4),5) However, the survival rate of HF remains poor, and the health burden from this condition is increasing globally.6),7),8),9),10),11),12),13) The impact of this condition has increased in Korea due to the increased growth and development of the society. The prevalence of risk factors such as diabetes, myocardial infarction, and ischemic heart disease has increased in the past few decades, although the survival outcomes from these diseases have also improved.14),15),16) Consequently, the prevalence of HF approximately doubled from 0.75% in 2002 to 0.53% in 2013, and the total medical cost increased by about 50% from 2009 to 2013.17),18) The increase in total medical cost was mostly attributable to the cost of in-hospital care. Unfortunately, the serial registry studies performed in Korea revealed that the survival from HF has not significantly improved during the past decades.11),19),20) This revealed an unmet need for a robust investigation of the demographic and clinical profiles, diagnostic and therapeutic approaches in routine practice, and the degree of adherence to clinical guidelines regarding pharmacological and non-pharmacological treatments. In addition, it also suggests the need for close examination of patients' clinical outcomes, prognostic factors, and trends over the last decade. Therefore, we established a robust registry of acute heart failure (AHF) in Korea and compared it with our previous registry.
Subjects and Methods
Patients and data collection
The Korean Acute Heart Failure (KorAHF) registry is a prospective multicenter cohort study designed to describe patient demographics, clinical characteristics, current treatments, and short-term and long-term patient outcomes of AHF. Detailed information on the study design and results from interim analysis are described in our previous paper.20) Briefly, patients who had signs or symptoms of HF and met one of the following criteria were eligible for this study: 1) lung congestion or 2) objective left ventricular systolic dysfunction or structural heart disease findings. Patients hospitalized for AHF from one of 10 tertiary university hospitals throughout the country were consecutively enrolled from March 2011 to February 2014. Follow-up of the patients is planned until 2018. Data were collected by each site and entered into a web-based case-report form in the web-based Clinical REsearch and Trial (iCreaT) system from the Korea National Institute of Health. Information about patient demographics, medical history, signs, symptoms, laboratory test results, electrocardiogram, echocardiography, medications, hospital course, and outcomes was collected at admission, at discharge, and during the follow-up (30-day, 90-day, 180-day, 1- to 5-year annually). In-hospital mortality and the mode of death were adjudicated by an independent event committee. The mortality data for patients who were lost to follow-up was collected from the National Insurance data or National Death Records. The study protocol was approved by the ethics committee/institutional review board at each hospital.
Variables and statistical analysis
Descriptive statistics are used to summarize demographic and clinical characteristics, clinical care during hospitalization, and patient outcomes. Detailed information on the variables was described in our previous paper.20) Data are reported as mean±standard deviation or median with range for continuous variables and as number (percentages) of patients for categorical variables. We used Student's t-test to demonstrate the statistical significance of differences between two groups if they showed a normal distribution and Wilcoxon rank sum test if they did not. Similarly, Chi-square test was used for categorical variables, while Fisher's exact test was used when 20% of the expected frequencies were less than 5. The individual participant data from the previous registry, the Korean Heart Failure (KorHF) registry, was received and approved by the KorHF writing committee. We extracted the data from 10 hospitals that participated in both the KorHF and KorAHF and summarized the demographic and clinical characteristics, clinical care during hospitalization, and outcomes based on descriptive statistics.
The logistic regression model was applied to verify predictors of in-hospital mortality. A binary model and multinomial model were used for all-cause mortality and cardiovascular death or non-cardiovascular death, respectively. Variables found to be statistically significant (p<0.1) in the univariable analysis were included in the multivariable model, except for variables with >10% missing values or variables that are closely related to other clinical variables and so may have multicollinearity issues. The analysis was performed using stepwise selection. The C-statistic was 0.865, and the p value for the Hosmer-Lemeshow goodness of fit test was 0.2669 for the binary multivariable logistic regression model, suggesting that our model is appropriate. For all statistical analyses, SAS software version 8.2 (SAS Institute Inc., Cary, NC, USA) and R version 3.2.5 were used. This project was supported by the Medical Research Collaborating Center at Seoul National University College of Medicine and Seoul National University Hospital.
Results
Demographic and clinical characteristics
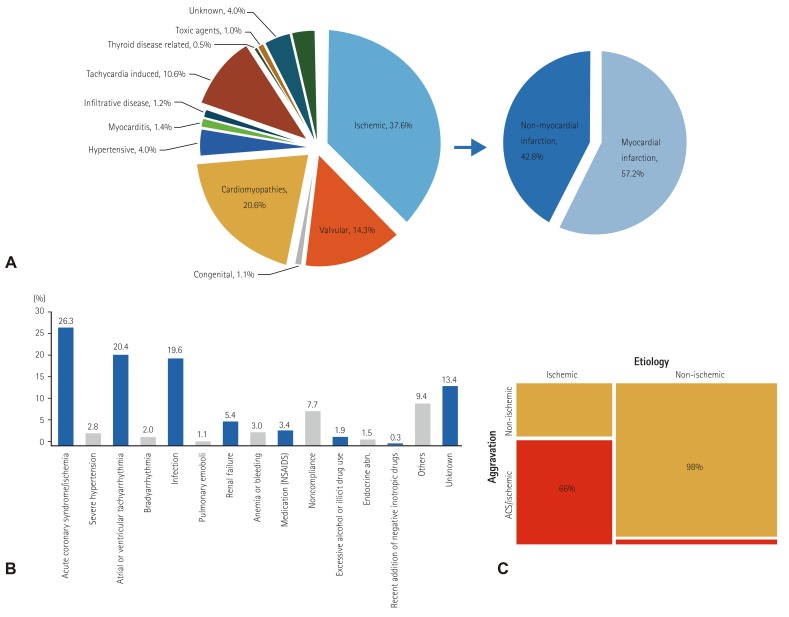
We enrolled 5625 AHF subjects from 10 tertiary university hospitals in Korea. The mean age was 68.5±14.5 years, and 53.2% were male (Table 1). In total 5103 of the enrolled patients were available for left ventricular ejection fraction (LVEF) measurement; 3088 had LVEF that was 40% or less, while 1285 had LVEF greater than 50%. More than half of the patients had de novo HF (n=2936, 52.2%). Hypertension was present in 62.2% of the patients, including 3.0% who were diagnosed with hypertension for the first time at the current admission; diabetes was present in 40.0%, including 4.7% newly diagnosed; ischemic heart disease (IHD) was found in 42.9% with 14.7% newly diagnosed, and chronic renal failure (CRF) was identified in 14.3%. Most of the patients had lung congestion (78.9%) and experienced dyspnea that was NYHA class III or IV (36.9% and 47.9%, respectively) at admission. The mean systolic and diastolic blood pressures (SBP and DBP) were 131.2±30.3 mmHg and 78.6±18.8 mmHg, respectively, and the mean pulse rate was 92.6±26.0 beats per minute at admission. The percentages of patients with hypotension (SBP <90 mmHg) and hypertension (SBP ≥140 mmHg) were 5.3% and 37.2%, respectively. Based on electrocardiogram (ECG) results, atrial fibrillation (AF) was present in 34.9% of patients at admission, whereas sustained ventricular tachycardia/fibrillation was found in 0.6% of patients. Pathological Q wave, right bundle branch block (RBBB), and left bundle branch block (LBBB) at ECG were present in 13.2%, 7.1%, and 5.2% of patients, respectively. Hyponatremia (serum sodium <135 mmEq/L) was present in 21.0% of patients, and the prevalence was significantly higher in patients with isolated RHF compared with other classifications of HF (27.1% vs. 20.9%, p=0.002). Azotemia (serum creatinine ≥2.0 mg/dL) was present in 14.9%, anemia (hemoglobin <12 mg/dL) in 42.3%, and leukocytosis (white blood cell [WBC] count ≥10000/mm3) in 27.2%. In total, 4842 (93.1%) patients had BNP ≥150 pg/mL or NT-proBNP ≥600 pg/mL. The laboratory results at admission and discharge are summarized in Table 2. The results indicate that IHD (37.6%) was the most frequent cause of HF, followed by idiopathic dilated cardiomyopathy (15.3%) and valvular heart disease (14.3%). Hypertension was found to be the cause in only 4.0% of patients (Fig. 1A). The most frequent causes of HF aggravation that led to admission were ischemia (26.3%), tachyarrhythmia (20.4%), and infection (19.6%), while a definitive cause of aggravation was not found in 13.4% of patients (Fig. 1B). Aggravating factors differed significantly based on HF etiology (Fig. 1C). In HF patients with non-ischemic causes, the aggravating factors were predominantly non-ischemic in origin. However, in HF patients with ischemic causes, both ischemic and non-ischemic factors were involved in HF aggravation.
Table 1. Baseline characteristics, clinical management, and outcomes.
| Characteristics | Total (n=5625) | Survivor (n=5356) | In-hospital death (n=269) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 68.5±14.5 | 68.4±14.5 | 70.5±14.4 | 0.013* |
| Male (%) | 53.2 | 53.0 | 58.4 | 0.083† |
| Body mass index (m/kg2) | 23.3±3.9 | 23.3±3.9 | 22.8±3.8 | 0.026* |
| Co-morbidities§ (%) | ||||
| Hypertension | 62.2 | 62.2 | 62.1 | 0.981† |
| Diabetes | 40.0 | 39.4 | 51.3 | <0.001† |
| Ischemic heart disease | 42.9 | 42.1 | 58.4 | <0.001† |
| Atrial fibrillation | 28.5 | 28.7 | 23.4 | 0.059† |
| Chronic lung disease | 11.3 | 11.0 | 15.6 | 0.021† |
| Cerebrovascular disease | 15.2 | 15.0 | 17.8 | 0.210† |
| Chronic renal failure | 14.3 | 14.0 | 20.8 | 0.002† |
| Etiology | ||||
| Ischemic CMP | 37.6 | 36.8 | 53.5 | <0.0001† |
| Hypertensive CMP | 4.0 | 4.1 | 1.5 | 0.034† |
| Idiopathic dilated CMP | 15.3 | 15.5 | 10.0 | 0.015† |
| Clinical status on admission and discharge | ||||
| De novo HF (%) | 52.2 | 52.3 | 49.8 | 0.423† |
| Lung congestion (%) | 78.9 | 78.3 | 90.3 | <0.001† |
| SBP at admission (mmHg) | 131.2±30.3 | 131.9±30.1 | 115.3±29.7 | <0.001* |
| SBP at discharge (mmHg) | 114.8±17.6 | 114.8±17.6 | - | - |
| DBP at admission (mmHg) | 78.6±18.8 | 79.0±18.6 | 69.9±20.2 | <0.001* |
| DBP at discharge (mmHg) | 67.1±11.5 | 67.1±11.5 | - | - |
| Heart rate at admission (/min) | 92.6±26.0 | 92.5±25.8 | 95.5±28.3 | 0.051* |
| Heart rate at discharge (/min) | 76.8±14.2 | 76.8±14.2 | - | - |
| NYHA class III-IV (%) at admission | 84.8 | 84.4 | 93.7 | <0.001† |
| NYHA class III-IV (%) at discharge | 10.8 | 10.8 | - | |
| ECG and echocardiography (%) | ||||
| RBBB | 7.1 | 6.8 | 12.6 | <0.001† |
| LBBB | 5.2 | 5.3 | 4.5 | 0.563† |
| Other IVCD | 6.2 | 5.7 | 14.9 | <0.001† |
| LVEF | 37.7±15.6 | 37.9±15.6 | 32.6±15.9 | <0.001* |
| Management (%) | ||||
| Parenteral diuretics | 74.9 | 74.4 | 85.9 | <0.001† |
| Parenteral inotropes | 31.1 | 28.4 | 85.1 | <0.001† |
| Parenteral vasodilators | 40.9 | 40.8 | 43.5 | 0.376† |
| ACEIs/ARBs at admission | 38.2 | 38.5 | 32.7 | 0.056† |
| ACEIs/ARBs at discharge | 65.9 | 68.8 | <0.001† | |
| Beta-blockers at admission | 28.3 | 28.6 | 22.7 | 0.035† |
| Beta-blockers at discharge | 49.9 | 52.2 | <0.001† | |
| AAs at admission | 18.8 | 18.8 | 17.8 | 0.695† |
| AAs at discharge | 44.9 | 46.6 | <0.001† | |
| Warfarin at discharge | 28.3 | 29.5 | <0.001† | |
| Heart transplantation | 1.2 | 1.2 | 2.6 | 0.049‡ |
| Outcomes | ||||
| Length of hospital stay (days) | 9 (1, 311) | 9 (1, 311) | 12 (1, 305) | <0.001* |
| Total cost per admission (US dollars)ll | 9672.2±20969.2 | 8682.3±17787.0 | 29462.5±50030.4 | <0.001* |
| Patient liability costs (US dollars) | 3047.5±6007.7 | 2843.8±5533.2 | 7119.1±11341.5 | <0.001* |
| In-hospital mortality (%) | 4.8 |
Values are presented as mean±standard deviation, median (min, max) or n (%). *A p value by Wilcoxon rank sum test, †p value by Chi-square test, ‡p value by Fisher's exact test, §includes in-hospital diagnoses, IIUS$ 1 is 1100 Korean won. CMP: cardiomyopathy, HF: heart failure, SBP: systolic blood pressure, DBP: diastolic blood pressure, ECG: electrocardiography, RBBB: right bundle branch block, LBBB: left bundle branch block, IVCD: intraventricular conduction delay, LVEF: left ventricular ejection fraction, ACEIs: angiotensin converting enzyme inhibitors, ARBs: angiotensin receptor blockers, AAs: aldosterone antagonists
Table 2. Laboratory tests on admission and discharge.
| Admission | Discharge | |||||
|---|---|---|---|---|---|---|
| Total | Survivor | In-hospital death | p* | Survivor | p | |
| WBC (/mL) | 8674.4±4081.3 | 8572.0±3993.8 | 10709.5±5142.7 | <0.001 | ||
| RDW (%) | 14.7±2.1 | 14.70±2.1 | 15.2±2.4 | 0.002 | ||
| Platelets (/mL) | 210534.0±88955.9 | 211429.8±86168.9 | 192747.2±131659.5 | <0.001 | ||
| HbA1c (%) | 6.8±1.4 | 6.8±1.4 | 6.6±1.3 | 0.511* | ||
| Total cholesterol (mg/dL) | 151.6±43.2 | 152.2±42.9 | 138.8±45.8 | <0.001* | ||
| Triglycerides (mg/dL) | 99.4±59.1 | 99.43±59.0 | 99.4±61.5 | 0.482* | ||
| HDL-cholesterol (mg/dL) | 41.5±13.9 | 41.7±13.8 | 35.0±15.8 | <0.001* | ||
| Albumin (g/dL) | 3.7±0.5 | 3.7±0.5 | 3.4±0.6 | <0.001* | ||
| Sodium (mmol/L) | 137.5±4.8 | 137.6±4.7 | 135.6±6.4 | <0.001* | 137.8±4.0 | <0.001† |
| Potassium (mmol/L) | 4.4±0.7 | 4.4±0.7 | 4.5±0.9 | <0.001* | 4.2±0.5 | <0.001† |
| Uric acid (mg/dL) | 7.1±2.9 | 7.0±2.9 | 7.9±3.2 | <0.001* | 6.7±2.7 | <0.001‡ |
| Hemoglobin (g/dL) | 12.4±2.3 | 12.4±2.3 | 12.0±2.3 | <0.001* | 12.0±2.1 | <0.001‡ |
| BUN (mg/dL) | 26.2±16.5 | 25.8±15.9 | 35.0±23.4 | <0.001* | 23.9±14.4 | <0.001‡ |
| Cr (mg/dL) | 1.5±1.5 | 1.5±1.5 | 1.9±1.7 | <0.001* | 1.4±1.3 | <0.001‡ |
| Glucose (mg/dL) | 155.4±76.9 | 154.2±75.8 | 180.6±92.5 | <0.001* | 117.3±46.2 | <0.001‡ |
| hs-CRP (mg/dL) | 2.3±4.2 | 2.2±4.0 | 4.0±5.8 | <0.001* | 2.1±3.1 | <0.001‡ |
| CRP (mg/dL) | 2.4±4.3 | 2.3±4.1 | 4.6±6.4 | <0.001* | 2.1±3.0 | <0.001‡ |
| BNP (pg/mL) | 1335.1±1301.5 | 1311.6±1287.5 | 1795.1±1484.3 | <0.001* | 848.8±1066.4 | <0.001‡ |
| NT-proBNP (pg/mL) | 9239.6±10802.4 | 8954.0±10612.8 | 14987.6±12839.2 | <0.001* | 6479.0±8898.6 | <0.001‡ |
| CK-MB (ng/mL) | 9.5±40.8 | 8.1±27.6 | 35.8±134.2 | <0.001* | 4.3±12.0 | <0.001‡ |
| TnI (ng/mL) | 2.9±19.9 | 2.3±17.7 | 13.2±42.1 | <0.001* | 2.2±13.3 | 0.142‡ |
| TnT (ng/mL) | 0.2±0.8 | 0.2±0.7 | 0.8±1.8 | <0.001* | 0.3±1.0 | 0.182‡ |
Values are presented as mean±standard deviation, median (min, max) or n (%). *A p value by Wilcoxon rank sum test between survivors and non-survivors, †p value by paired t-test between survivor levels at admission and discharge, ‡p value by Wilcoxon signed rank test between survivor levels at admission and discharge. WBC: white blood cells, RDW: red cell distribution width, HbA1c: hemoglobin A1c, HDL: high density lipoprotein, BUN: blood urea nitrogen, Cr: creatinine, hs-CRP: high sensitivity C-reactive protein, CRP: C-reactive protein, BNP: brain natriuretic peptides, NT-proBNP: N-terminal pro-brain natriuretic peptides, CK-MB: creatine kinase-MB, TnI: troponin I, TnT: troponin T
Fig. 1. Etiologies and aggravating factors of acute heart failure. (A) Etiologies of acute heart failure, (B) aggravating factors of acute heart failure, (C) aggravating factors in ischemic and non-ischemic cardiomyopathies. acute coronary syndrome. NSAIDs: non-steroidal anti-inflammatory drugs, ACS: acute coronary syndrome.
Management during hospitalization
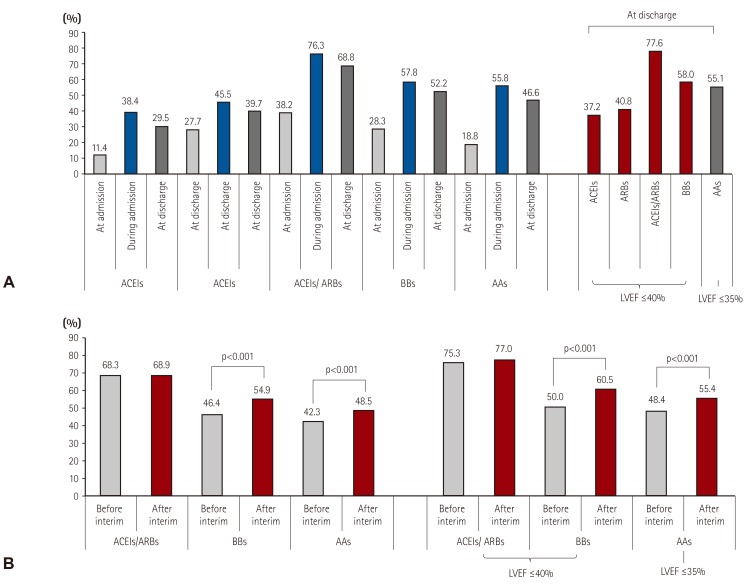
The proportion of patients who received evidence-based HF medications increased from the point of admission to discharge, and 68.8%, 52.2%, and 46.6% of patients were on angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), beta-blockers, or aldosterone antagonists (AAs) at discharge, respectively. Following the indication that ACEIs, ARBs, and beta-blockers are recommended when LVEF is ≤40% and AAs when LVEF is ≤35%,21) 77.6%, 58.0% and 55.1% of the patients were treated with ACEIs/ARBs, beta-blockers, or AAs at discharge, respectively (Fig. 2A). ARBs were more frequently prescribed than ACEIs (ARBs 51.9% vs. ACEIs 47.4%), and only 0.7% of the population received both medications. Among those treated with beta-blockers, 60.8% of patients received carvedilol, 32.5% bisoprolol, and 3.0% nebivolol. Warfarin was used in 28.3% of the overall population and in 51% of the patients with atrial fibrillation. Loop diuretics were prescribed for 91.6% of patients during admission, primarily through the parenteral route (74.9%). Parenteral inotropes were used in 31.1% of patients and parenteral vasodilators in 40.9% of patients. Of the total patients, 15.3% received mechanical ventilation care, and 7.5% received renal replacement therapy. Red blood cells were transfused in 21.4% of patients. Percutaneous coronary intervention and coronary artery bypass graft surgery were performed in 10.8% and 2.3% of patients, respectively, and an assist device was inserted in 5.9% of patients. Detailed pharmacological and non-pharmacological treatments during admission are described in Table 3.
Fig. 2. Evidence-based medication prescriptions. (A) Prescription rate of angiotensin converting enzyme inhibitors: angiotensin receptor blockers: beta-blockers and aldosterone antagonists for acute heart failure patients. (B) Prescription rate changes in evidence-based medication before and after interim analysis. LVEF: left ventricular ejection fraction, ACEIs: angiotensin converting enzyme inhibitor, ARBs: angiotensin receptor blockers, BBs: beta-blockers, AAs: aldosterone antagonist.
Table 3. Hospital treatment.
| Total | Survivor | In-hospital death | p | |
|---|---|---|---|---|
| Pharmacological treatments | ||||
| Nitrates | ||||
| Sustained use | 1231 (21.9) | 1206 (22.5) | 25 (9.3) | <0.001* |
| Transient use | 1893 (33.7) | 1777 (33.2) | 116 (43.1) | |
| Hydralazine | ||||
| Sustained use | 32 (0.6) | 32 (0.60) | 0 (0.0) | 0.102† |
| Transient use | 63 (1.1) | 57 (1.1) | 6 (2.2) | |
| Loop diuretics | ||||
| Sustained use | 3991 (71.0) | 3895 (72.7) | 96 (35.7) | <0.001* |
| Transient use | 1163 (20.7) | 1014 (18.9) | 149 (55.4) | |
| Thiazide diuretics | ||||
| Sustained use | 457 (8.1) | 451 (8.4) | 6 (2.2) | 0.001* |
| Transient use | 321 (5.7) | 304 (5.7) | 17 (6.3) | |
| Amiodarone | ||||
| Sustained use | 422 (7.5) | 382 (7.1) | 40 (14.9) | <0.001* |
| Transient use | 439 (7.8) | 367 (6.9) | 72 (26.8) | |
| Digoxin | ||||
| Sustained use | 1437 (25.6) | 1402 (26.2) | 35 (13.0) | <0.001* |
| Transient use | 456 (8.1) | 394 (7.4) | 62 (23.1) | |
| Heparin/LMWH | ||||
| Sustained use | 85 (1.5) | 34 (0.6) | 51 (19.0) | <0.001* |
| Transient use | 2523 (44.9) | 2383 (44.5) | 140 (52.0) | |
| Warfarin | ||||
| Sustained use | 1591 (28.3) | 1580 (29.5) | 11 (4.1) | <0.001* |
| Transient use | 213 (3.8) | 186 (3.5) | 27 (10.0) | |
| Aspirin | ||||
| Sustained use | 3019 (53.7) | 2948 (55.0) | 71 (26.4) | <0.001* |
| Transient use | 607 (10.8) | 512 (9.6) | 95 (35.3) | |
| Statins | ||||
| Sustained use | 2328 (41.4) | 2286 (42.7) | 42 (15.6) | <0.001* |
| Transient use | 273 (4.9) | 209 (3.9) | 64 (23.8) | |
| Ivabradine | ||||
| Sustained use | 4 (0.1) | 4 (0.1) | 0 (0.0) | 1.000† |
| Transient use | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Dronedarone | ||||
| Sustained use | 3 (0.1) | 3 (0.1) | 0 (0.0) | 1.000† |
| Transient use | 5 (0.1) | 5 (0.1) | 0 (0.0) | |
| Insulin | 1285 (22.8) | 1159 (21.6) | 126 (46.8) | <0.001* |
| Oral diabetes medication | 1319 (23.5) | 1290 (24.1) | 29 (10.8) | <0.001* |
| Parenteral medications | ||||
| Diuretics | 4214 (74.9) | 3983 (74.4) | 231 (85.9) | <0.001* |
| Dobutamine | 1279 (22.7) | 1100 (20.5) | 179 (66.5) | <0.001* |
| Dopamine | 986 (17.5) | 791 (14.8) | 195 (72.5) | <0.001* |
| Milrinone | 141 (2.5) | 123 (2.3) | 18 (6.7) | <0.001* |
| Norepinephrine | 528 (9.4) | 386 (7.2) | 142 (52.8) | <0.001* |
| Nitroprusside | 60 (1.1) | 55 (1.0) | 5 (1.9) | 0.210† |
| Nitroglycerin | 2280 (40.5) | 2163 (40.4) | 117 (43.5) | 0.311* |
| Non-pharmacological treatments | ||||
| Blood transfusion | 1203 (21.4) | 1033 (19.3) | 170 (63.2) | <0.001* |
| Mechanical ventilation | 862 (15.3) | 688 (12.9) | 174 (64.7) | <0.001* |
| Assisting device | 333 (5.9) | 234 (4.4) | 99 (36.8) | <0.001* |
| IABP | 198 (3.5) | 143 (2.7) | 55 (20.5) | <0.001* |
| LVAD | 4 (0.1) | 0 (0.0) | 4 (1.5) | <0.001† |
| ECMO/PCPS | 155 (2.8) | 87 (1.6) | 68 (25.3) | <0.001* |
| Renal replacement | 423 (7.5) | 297 (5.6) | 126 (46.8) | <0.001* |
| CRT | 45 (0.8) | 40 (0.8) | 5 (1.9) | 0.062† |
| ICD | 73 (1.3) | 71 (1.3) | 2 (0.7) | 0.584† |
| PCI | 605 (10.8) | 557 (10.4) | 48 (17.8) | <0.001* |
| CABG | 130 (2.3) | 120 (2.2) | 10 (3.7) | 0.116* |
| Valve operation | 183 (3.3) | 174 (3.3) | 9 (3.4) | 0.930* |
| Heart transplantation | 70 (1.2) | 63 (1.2) | 7 (2.6) | 0.049† |
*A p value by Chi-square test, †p value by Fisher's exact test. LMWH: low-molecular weight heparin, IABP: intra-aortic balloon pump, LVAD: left ventricular assistant device, ECMO: extracorporeal membrane oxygenation, PCPS: percutaneous cardiopulmonary support, CRRT: continuous renal replacement therapy, CRT: cardiac resynchronization therapy, ICD: implantable cardioverter defibrillator, PCI: percutaneous coronary intervention, CABG. coronary artery bypass graft
Predictors and outcomes
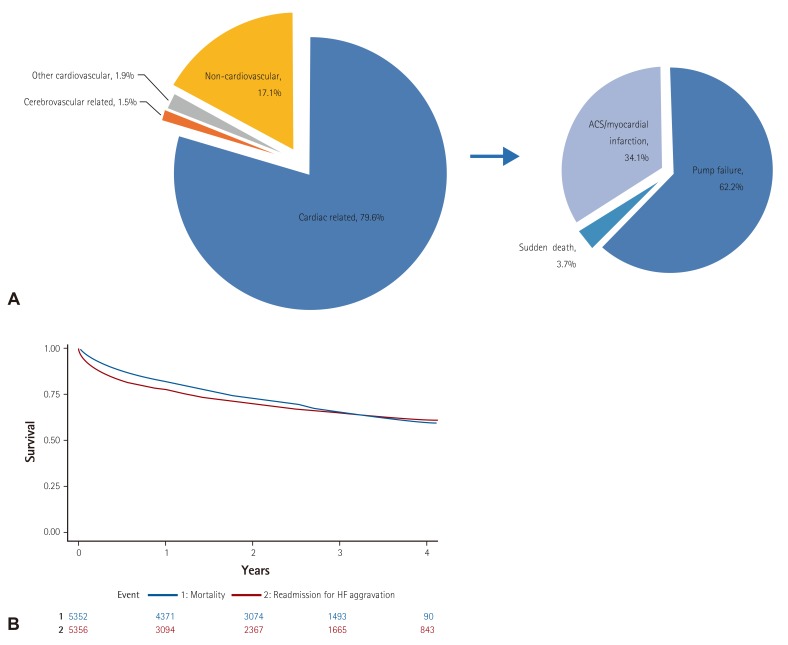
In-hospital mortality was 4.8%. Heart transplantation was performed in 70 patients (1.2%), 7 of whom died during hospitalization. The median duration of hospitalization was 9 days. After adjudication, 79.6% died due to cardiac problems; among them, 62.2% were due to pump failure, 3.7% due to sudden cardiac death, and 34.1% due to acute coronary syndrome or myocardial infarction (Fig. 3A). Sepsis and pneumonia were the most frequent causes of non-cardiovascular death (37.0% and 28.3%, respectively). There were significant differences in demographic and clinical characteristics and hospital course between survivors and non-survivors (Table 1). The patients that died were characterized by advanced age (deceased 70.5±14.4 years vs. survivors 68.4±14.5 years, p=0.013), lower body mass index (body mass index 22.8±3.8 vs. 23.3±3.9, p=0.026), higher diabetes prevalence (51.3% vs. 39.4%, p<0.001), and more frequent IHD (58.4% vs. 42.1%, p<0.001) and CRF (20.8% vs. 14.0%, p<0.001). They had lower blood pressure (SBP 115.3±29.7 mmHg vs. 131.9±30.1 mmHg, p<0.001) and more severe symptoms at admission (NYHA class III-IV 93.7% vs. 84.4%, p<0.001). The deceased also had lower LVEF (32.6±15.9% vs. 37.9±15.6%, p<0.001), stayed longer in the hospital (median 12 days vs. 9 days, p<0.001), and had an approximately four times higher cost of medical care than those that were discharged. A multivariate logistic regression analysis revealed that acute or chronic renal failure at admission, lower SBP at admission (<100 mmHg), ischemia as an aggravating factor, Q wave, right bundle branch block (RBBB) or other intraventricular conduction delay at ECG, advanced age (≥70 years), hyponatremia (<135 mmEq/L), and leukocytosis (≥10000/mm3) at admission were independently related with worsening outcomes, while higher body mass index (BMI ≥25 m/kg2) and prior use of beta-blockers were independent predictors of lower in-hospital mortality (Table 4). NYHA functional class III or IV at admission and LVEF no more than 40% were also associated with in-hospital mortality caused by cardiovascular problems. Infection as an aggravating factor was a unique predictor of non-cardiovascular death. Among those discharged, post-discharge 30-day mortality was 3.3%, 90-day mortality was 8.4%, 180-day mortality was 12.4%, 1-year mortality was 18.2%, 2-year mortality was 27.6%, and 3-year mortality was 34.7% during a median follow-up of 2.2 years. Post-discharge re-hospitalization for HF aggravation at 30-days, 90-days, 180-days, 1-year, 2-years, and 3-years was 7.0%, 13.5%, 17.9%, 23.1%, 30.3%, 36.0% during a median follow-up of 1.5 years, respectively (Fig. 3B).
Fig. 3. Clinical outcomes of acute heart failure. (A) Mechanism of in-hospital mortality, (B) Kaplan-Meier survival curve for all-cause mortality (blue) and re-hospitalization due to heart failure aggravation (red) after discharge. HF: heart failure.
Table 4. Multivariable logistic regression for in-hospital mortality and multinomial logistic regression for cardiovascular or non-cardiovascular death.
| All-cause death | Cardiovascular death | Non-cardiovascular death | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI)1) | p | Adjusted OR (95% CI)1) | p | |
| Elderly (≥70 years) | 1.593 (1.138:2.231) | 0.007 | ||||
| Body mass index (≥25 kg/m2) | 0.609 (0.410:0.906) | 0.014 | 0.649 (0.417:1.009) | 0.055 | 0.435 (0.190:0.998) | 0.049 |
| SBP (<100 mmHg) | 2.447 (1.688:3.546) | <0.001 | 2.331 (1.558:3.497) | <0.001 | 2.242 (1.081:4.651) | 0.030 |
| NYHA III or IV | 2.658 (1.145:6.171) | 0.023 | 0.423 (0.194:0.920) | 0.030 | ||
| Chronic renal failure | 5.67 (3.656:8.794) | <0.001 | 4.770 (2.903:7.835) | <0.001 | 8.830 (3.555:21.930) | <0.001 |
| Acute renal failure at admission | 13.315 (9.09:19.503) | <0.001 | 12.236 (7.995:18.727) | <0.001 | 19.234 (8.413:43.978) | <0.001 |
| Ischemia as an aggravating factor | 1.585 (1.131:2.222) | 0.008 | 2.082 (1.413:3.068) | <0.001 | 1.142 (0.541:2.410) | 0.727 |
| Infection as an aggravating factor | 1.388 (0.893:2.158) | 0.145 | 2.906 (1.512:5.585) | 0.001 | ||
| Beta-blockers before admission | 0.657 (0.453:0.951) | 0.026 | ||||
| Q wave at ECG | 2.067 (1.409:3.034) | <0.001 | 2.296 (1.522:3.465) | <0.001 | 1.024 (0.387:2.706) | 0.962 |
| RBBB at ECG | 1.781 (1.082:2.930) | 0.023 | ||||
| Other IVCD at ECG | 2.695 (1.699:4.275) | <0.001 | 2.216 (1.322:3.713) | 0.003 | 3.304 (1.366:7.993) | 0.008 |
| Leukocytosis | 1.6 (1.153:2.219) | 0.005 | 1.461 (1.011:2.113) | 0.049 | 1.731 (0.918:3.265) | 0.090 |
| Hyponatremia | 1.806 (1.303:2.503) | <0.001 | 1.835 (1.274:2.646) | 0.001 | 1.319 (0.688:2.532) | 0.405 |
| LVEF ≤40% | 1.560 (1.025:2.375) | 0.038 | 0.550 (0.288:1.049) | 0.070 | ||
| C-statistics | 0.865 | |||||
OR: odds ratio, CI: confidential interval, SBP: systolic blood pressure, ECG: electrocardiography, RBBB: right bundle branch block, IVCD: intraventricular conduction delay, Leukocytosis: white blood cell count ≥10000/mm3, Hyponatremia: serum sodium <135 mmEq/L, LVEF: left ventricular ejection fraction
Discussion
Patient demographics, clinical profiles, and AHF management and outcomes
We compared the results of this study with previous data from the KorHF, which also had enrolled patients that were hospitalized for AHF in Korea, to assess trends in demographic characteristics, clinical profiles, and management and outcomes of AHF in Korea over the last decade.11) The KorHF study enrolled a total of 3200 patients from 24 hospitals from June 2004 to April 2009 and had an average follow-up of 1.7 years. We extracted data from the hospitals that had participated both previously and currently in order to describe temporal trends and to limit possible bias that could be caused by differences among hospitals (Table 5). The age became older and there were more co-morbidities such as hypertension, diabetes and chronic lung disease. More patients had history of HF. Clinical profiles at admission were similar except for more atrial fibrillation presented at admission. There was no significant difference in laboratory tests results. In terms of management, more parenteral drugs have been used, and guidelines for these prescriptions have improved. The most significant changes were seen for frequent use of extracorporeal membrane oxygenation (ECMO) or percutaneous cardiopulmonary support (PCPS) and heart transplantation. The in-hospital mortality was markedly reduced over the past decade, while the costs have increased by about 40%. In contrast, one-year mortality significantly increased in our registry compared to the previous registry. However, it was difficult to directly compare post-discharge follow-up outcomes between the two registries, because our registry validated most of the patient survival with the National Insurance data or National Death Records mortality (except for 20 patients, including foreigners), while the previous registry had not validated mortality, which could have resulted in an underestimation of the mortality rate. We assessed the impact of mortality validation on in-hospital mortality rate in one participating hospital and found that the 6-month mortality rate increased from 10.7% to 15% after validation in patients that did not complete follow-up due to death. Similarly, our interim analysis, published before the mortality validation, reported that the 6-month mortality rate was 9.2%, which increased to 12.5% in our final analysis after the validation. Thus, we concluded that, over the past decade, there has been significant improvement in acute clinical care and AHF-related outcomes, although the improvements have not yet impacted long-term mortality rates in Korea.
Table 5. KorAHF and KorHF comparisons.
| KorAHF (2011–2014) (n=5625) | KorHF (2004–2009) (n=1788)* | |
|---|---|---|
| Demographics | ||
| Age (years) | 68.5±14.5 | 66.5±15.1 |
| Male (%) | 53.2 | 52.3 |
| Body mass index (m/kg2) | 23.3±3.9 | 23.2±4.0 |
| Co-morbidities† (%) | ||
| Hypertension | 59.1 | 48.7 |
| Diabetes | 35.3 | 29.9 |
| Previous MI | 16.8 | 16.5 |
| Previous HF history | 43.7 | 37.5 |
| Chronic lung disease | 11.3 | 4.8 |
| Cerebrovascular disease | 15.2 | 17.9 |
| Chronic renal failure | 14.3 | 12.5 |
| Etiology and aggravation (%) | ||
| Ischemic CMP | 37.6 | 35.9 |
| Idiopathic dilated DCMP | 15.3 | 21.7 |
| Unknown etiology | 4.0 | 8.5 |
| Aggravation by ischemia | 26.3 | 28.8 |
| Aggravation by hypertension | 2.8 | 5.8 |
| Clinical status on admission and discharge | ||
| SBP at admission (mmHg) | 131.2±30.3 | 132.0±30.1 |
| DBP at admission (mmHg) | 78.6±18.8 | 78.4±18.6 |
| Heart rate at admission (/min) | 92.6±26.0 | 91.7±25.4 |
| NYHA class III-IV (%) | 84.8 | 84.6 |
| Atrial fibrillation at admission (%) | 34.9 | 25.6 |
| LVEF (%) | 37.7±15.6 | 37.9±15.2 |
| Laboratory tests at admission | ||
| White blood cell count (/mL) | 8674.4±4081.3 | 9417.6±4527.9 |
| Hemoglobin (g/dL) | 12.4±2.3 | 12.5±2.4 |
| Serum creatinine (mg/dL) | 1.5±1.5 | 1.5±1.5 |
| Serum sodium (mmol/L) | 137.5±4.8 | 137.3±5.1 |
| BNP (pg/mL) | 1335.1±1301.5 | 1336.7±1977.2 |
| NT-proBNP (pg/mL) | 9239.6±10802.4 | 8964.4±9962.1 |
| LBBB at ECG (%) | 7.1 | 6.4 |
| RBBB at ECG (%) | 5.2 | 5.4 |
| Management (%) | ||
| Parenteral diuretics | 74.9 | 63.9 |
| Parenteral inotropes | 31.1 | 24.0 |
| Parenteral vasodilators | 40.9 | 37.2 |
| ACEIs/ARBs at discharge | 68.8 | 68.8 |
| Beta-blockers at discharge | 52.2 | 43.7 |
| AAs at discharge | 46.6 | 37.1 |
| ECMO/PCPS | 2.8 | 0.8 |
| Heart transplantation | 1.2 | 0.3 |
| Outcomes | ||
| Total costs for hospital care‡ (US dollars) | 9672.2±20969.2 | 6805.5±12146.8 |
| Patient liability costs‡ (US dollars) | 3047.5±6007.7 | 2483.4±4726.3 |
| Length of stay (day) | 9 (1, 311) | 9 (1, 403) |
| In-hospital mortality (%) | 4.8 | 7.6 |
| One-year follow-up mortality (%) | 18.2 | 8.4§ |
Values are presented as mean±standard deviation or number (%). *Population of hospitals participating KorAHF, †from past medical history, ‡US$ 1 is 1100 Korean won, §mortality data was not validated in all patients. KorAHF: Korean acute heart failure, KorHF: Korean heart failure, MI: myocardial infarction, HF: heart failure, CMP: cardiomyopathy, SBP: systolic blood pressure, DBP: diastolic blood pressure, LVEF: left ventricular ejection fraction, BNP: brain natriuretic peptides, NT-proBNP: N-terminal pro-brain natriuretic peptides, RBBB: right bundle branch block, LBBB: left bundle branch block, ECG: electrocardiography, ACEIs: angiotensin converting enzymes, ARBs: angiotensin receptor blockers, AAs: aldosterone antagonists, ECMO: extracorporeal membrane oxygenation, PCPS: percutaneous cardiopulmonary support
AHF patient profiles in Korea
Our registry with final enrollment confirms that lower blood pressure at admission is a unique characteristic of AHF in Korea, and this result was previously suggested by our interim analysis. Initial blood pressure at admission was lower than that measured by other registries: the mean SBP at admission was 131 mmHg in our registry, while it was 147 mmHg in ATTEND,6) 144 mmHg in ADHERE,22) and 143 mmHg in OPTIMIZE-HF.8) Hypertension was the most common co-morbidity of HF, but it was less frequently combined in our patients compared with those in other registries (62% vs. 71% in ATTEND, 74% in ADHERE, 71% in OPTIMIZE-HF, 63% in EHFSII.23) The prevalence of HF with hypertension as an etiology (4%) was also lower than in other registries, such as ATTEND (18%), OPTIMIZE-HF (23%), and EHFSII (11%). Another interesting feature of AHF patients in Korea was the prevalence of bundle branch block (BBB) on ECG. Our interim analysis revealed that only a small proportion of patients had received cardiac resynchronization therapy (CRT). We investigated the reason for this and found that the prevalence of both LBBB and RBBB was lower in our patients compared with those in Western countries. After adjudication by a cardiologist, we determined that the prevalence of LBBB and RBBB were 7.1% and 5.2%, respectively, in our registry. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry reported that 16.7% and 10.6% patients had LBBB and RBBB, respectively.24) Among those admitted to the intensive care unit at Henry Ford Hospital in the United States, 13.2% had LBBB and 7.3% had RBBB,25) among those hospitalized for HF in UK hospitals, 15% had LBBB and 7% had RBBB.26) This lower prevalence of BBB in Korea might partially explain the underused CRT in Korea. These unique features of AHF in Korea were also supported by the analysis of the KorHF population (Table 5).
Medical therapy and guideline adherence for HF patients
The unique characteristics of our patients, particularly characteristics related to blood pressure, could affect the use of evidence-based medications, such as ARBs, ACEIs, beta-blockers, AAs, and other drugs, such as parenteral diuretics and inotropes. As demonstrated by interim analysis, there was less frequent use of ACEIs/ARBs in our registry compared with other registries (68.8% vs. 83% in ADHERE, 80% in EHFSII, and 77% in the ESC-HF Long-Term registry).27) The less frequent prescription of beta-blockers was more remarkable. In our registry, beta-blockers were prescribed in 52.2% of cases while they were prescribed in 80% of ADHERE cases, 61% in EHFSII, and 71.8% in the ESC-HF Long-Term registry. Although Asians are often reported to have higher sensitivity to beta-blockers compared to other populations, 64% of Korean HF patients are reported to have active adrenalin receptor 1 polymorphism, which requires a 33% increased dose of beta blocker to achieve the same range of heart rate reduction compared with the less active genotype.28) The prescription rate of AAs was moderate (46.6% in KorAHF vs. 33% in ADHERE, 48% in EHFSII, 55.3% in the ESC-HF Long-Term registry). However, the statistics from these studies do not directly infer a problem with guideline adherence in Korea, since the indications and contraindications for these evidence-based medications might differ from registry to registry. For example, if we consider LVEF as an indication of the prescription of neurohormonal blockages and exclude those patients who received heart transplant, the prescription rates of ACEIs or ARBs, beta-blockers, and AAs would increase to 77.6%, 58.0%, and 55.1%, respectively. Thus, the degree of adherence might be much greater when we consider the contraindications of ACEIs/ARBs or beta-blockers, such as lower blood pressure, renal failure, or chronic obstructive lung diseases. An interesting finding was that the prescription rate of evidence-based medical therapy has increased significantly after the results of our interim analysis were released, and the interim analysis indicated that the prescription rate of evidence-medications in Korea is lower than that in other registries (Fig. 2B). This implies that establishing a registry, objectively and comprehensively assessing current practice patterns, and sharing results among clinicians could improve performance.
Study limitations
There were several limitations to this study. First, the KorAHF is not a clinical trial but a patient registry. Therefore, assessment, management, and follow-up regimens have not been standardized and vary by institution. Unmeasured variables may have influenced the results. Second, because only tertiary educational hospitals participated in this registry and were not selected based on statistical measures according to population dynamics, our cohort might not represent the general population of HF patients in Korea. Third, while in-hospital mortality was confirmed by an independent events committee, the causes of post-discharge mortality and re-hospitalization were not validated. Because this result is different than that of all-cause mortality, there are missing values for re-hospitalization statistics. Finally, laboratory tests and echocardiographic results were not centralized.
Conclusion
In conclusion, our analysis of AHF patients in Korea demonstrates that Korean patients have lower blood pressure and lower BBB prevalence at admission, which could possibly affect the clinical practice pattern and the degree of adherence to current clinical guidelines. Invasive treatments such as ECMO/PCPS and heart transplantation have been performed more frequently compared to past studies, which showed increased acute AHF-related clinical outcomes, which remain to be addressed. However, the long-term prognosis of HF is still poor, and the burden of medical costs remains high. Our study suggests the need for further rigorous studies to determine the most cost-effective approach for HF management and to better assess the long-term HF-related outcomes using critical examination of current clinical practice patterns. A comprehensive study will ultimately help establish a patient-friendly clinical care system for HF in Korea.
Acknowledgments
This work was supported by the Research of Korea Centers for Disease Control and Prevention [2010-E63003-00, 2011-E63002-00, 2012-E63005-00, 2013-E63003-00, 2013-E63003-01, 2013-E63003-02, and 2016-ER6303-00].
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Okura Y, Ramadan MM, Ohno Y, et al. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72:489–491. doi: 10.1253/circj.72.489. [DOI] [PubMed] [Google Scholar]
- 5.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 6.Sato N, Kajimoto K, Asai K, et al. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J. 2010;159:949–955.e1. doi: 10.1016/j.ahj.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW ADHERE Scientific Advisory Committee and Investigators. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153:1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 10.West R, Liang L, Fonarow GC, et al. Characterization of heart failure patients with preserved ejection fraction: a comparison between ADHERE-US registry and ADHERE-International registry. Eur J Heart Fail. 2011;13:945–952. doi: 10.1093/eurjhf/hfr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi DJ, Han S, Jeon ES, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the korean heart failure registry. Korean Circ J. 2011;41:363–371. doi: 10.4070/kcj.2011.41.7.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis GS, Cogswell R, Thenappan T. The heterogeneity of heart failure: will enhanced phenotyping be necessary for future clinical trial success? J Am Coll Cardiol. 2014;64:1775–1776. doi: 10.1016/j.jacc.2014.07.978. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 14.Kook HY, Jeong MH, Oh S, et al. Current trend of acute myocardial infarction in Korea (from the Korea Acute Myocardial Infarction Registry from 2006 to 2013) Am J Cardiol. 2014;114:1817–1822. doi: 10.1016/j.amjcard.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Ha KH, Kim DJ. Trends in the diabetes epidemic in Korea. Endocrinol Metab (Seoul) 2015;30:142–146. doi: 10.3803/EnM.2015.30.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jhun HJ, Kim H, Cho SI. Time trend and age-period-cohort effects on acute myocardial infarction mortality in Korean adults from 1988 to 2007. J Korean Med Sci. 2011;26:637–641. doi: 10.3346/jkms.2011.26.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016;46:658–664. doi: 10.4070/kcj.2016.46.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Service KNHI [Internet] 2014. Sep, [cited 2016 October]. Available from: http://www.nhis.or.kr/bbs7/boards/B0039/3171.
- 19.Han SW, Ryu KH, Chae SC, et al. Multicenter analysis of clinical characteristics and prognostic factors of patients with congestive heart failure in Korea. Korean Circ J. 2005;35:357–361. [Google Scholar]
- 20.Lee SE, Cho HJ, Lee HY, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 21.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 22.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Harjola VP, Follath F, Nieminen MS, et al. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–248. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 24.Chioncel O, Vinereanu D, Datcu M, et al. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J. 2011;162:142–153.e1. doi: 10.1016/j.ahj.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 25.McCullough PA, Hassan SA, Pallekonda V, et al. Bundle branch block patterns, age, renal dysfunction, and heart failure mortality. Int J Cardiol. 2005;102:303–308. doi: 10.1016/j.ijcard.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Farwell D, Patel NR, Hall A, Ralph S, Sulke AN. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246–1250. doi: 10.1053/euhj.1999.1985. [DOI] [PubMed] [Google Scholar]
- 27.Maggioni AP, Anker SD, Dahlström U, et al. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC heart failure long-term registry. Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 28.Lee HY, Chung WJ, Jeon HK, et al. Impact of the β-1 adrenergic receptor polymorphism on tolerability and efficacy of bisoprolol therapy in Korean heart failure patients: association between β adrenergic receptor polymorphism and bisoprolol therapy in heart failure (ABBA) study. Korean J Intern Med. 2016;31:277–287. doi: 10.3904/kjim.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]