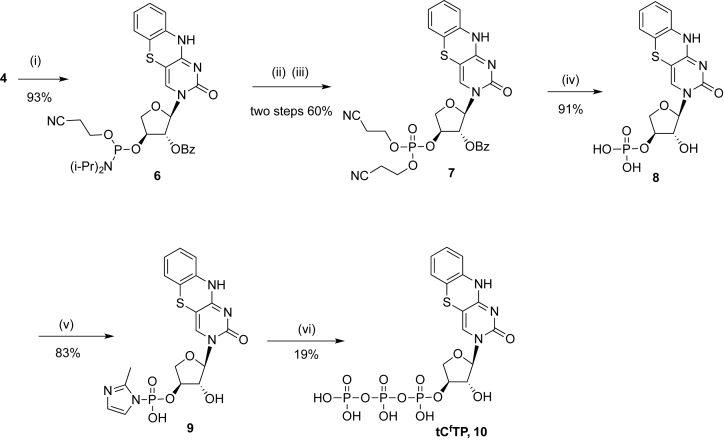
Scheme 2.
aReagents and conditions. (i) NC(CH2)2OP(Cl)N(i-Pr)2, (i-Pr)2EtN, DMAP, rt, 40 min; (ii) 3-hydroxypropionitrile, tetrazole, CH3CN, rt, 3h; (iii) H2O2, THF, rt. 20 min; (iv) NH4OH, 37°C, 16 h, ; (v) 2-methylimidazole, PPh3, 2,2΄-dipydiyl disulfide, triethylamine, DMF–DMSO, rt, 6 h; (vi) tributylammonium pyrophosphate, tributylamine, anhydrous DMF, rt, 6 h.