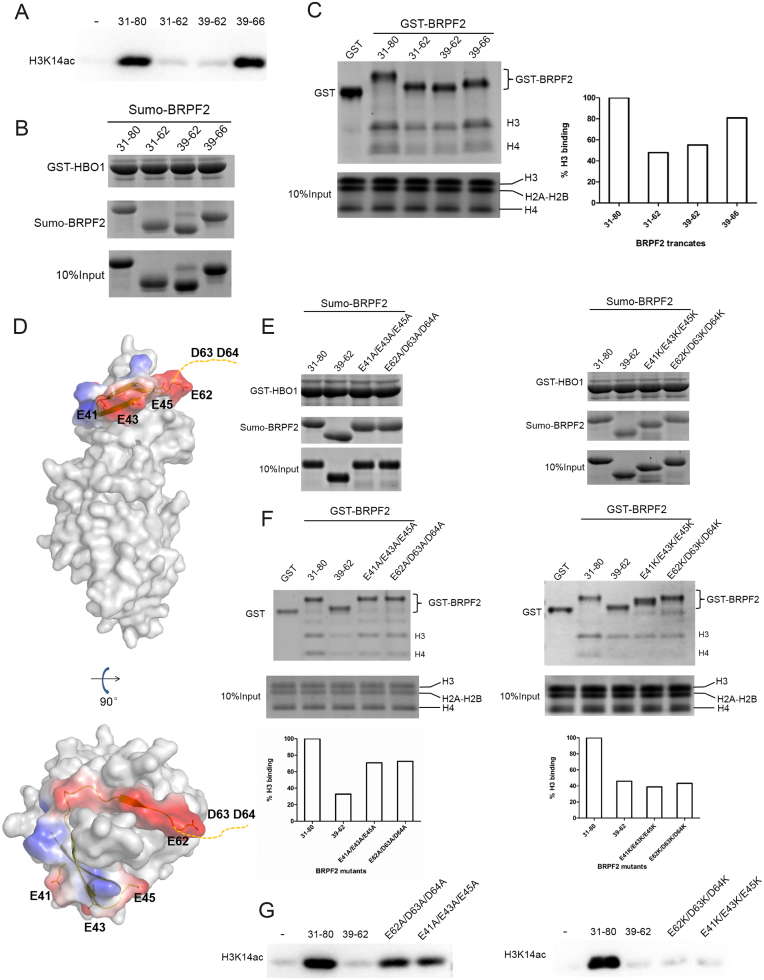
Figure 4.
Examination of potential binding of the N-terminal region of BRPF2 with histones in vitro. (A) In vitro HAT activity assays of HBO1 toward H3K14 in the presence of different Sumo–BRPF2 fragments. (B) In vitro GST pull-down assays of GST–HBO1 with different Sumo–BRPF2 fragments. Equal loading of Sumo–BRPF2 is shown. (C) In vitro GST pull-down assays of different GST–BRPF2 fragments with free H3 and H4 from histone mixture. The amount of histone H3 that was pulled down was semi-quantitated. (D) Two negatively charged surface patches in the N-terminal region of BRPF2. HBO1 is shown with gray surface. BRPF2 is shown with electrostatic surface and the conserved acidic residues are shown with side chains. Asp63 and Asp64 are invisible in the HBO1–BRPF2 structure and thus are indicated with a dash line. (E) In vitro GST pull-down assays of GST–HBO1 with WT or mutant Sumo–BRPF2 (31–80). Equal loading of Sumo–BRPF2 is shown. (F) In vitro GST pull-down assays of WT or mutant GST–BRPF2 (31–80) with free H3 and H4 from histone mixture. The amount of histone H3 that was pulled down was semi-quantitated. (G) In vitro HAT activity assays of HBO1 toward H3K14 in the presence of WT or mutant Sumo–BRPF2 (31–80).