FIGURE 3.
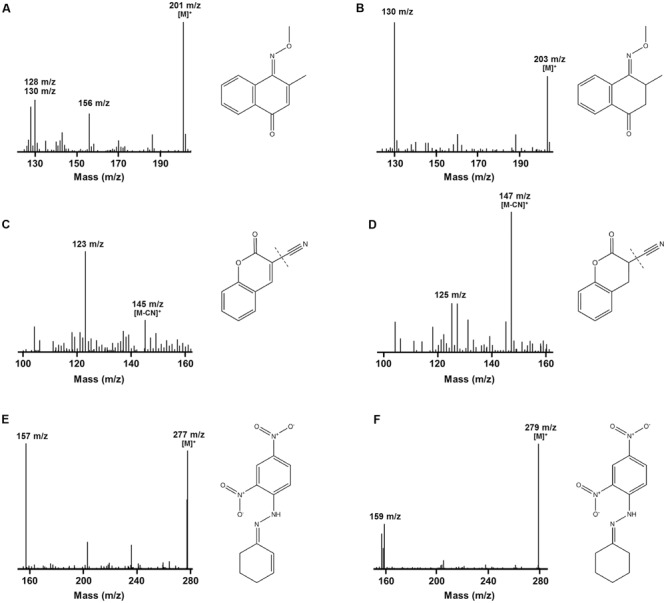
Mass spectra infer that F420H2-dependent reductases mediate hydrogenation of substrates. The spectra show the detection of substrate standards and reaction products following reduction with MSMEG_2027. GC/MS spectra of the single methoxime derivatives of the (A) menadione standard and (B) 2,3-dihydromenadione product. Mass spectra of the underivatized compounds are shown in Supplementary Figure S3. Keto–enol tautomerization is likely to result in menadiol formation under physiological conditions. LC/MS spectra of the (C) 3-cyanocoumarin standard and (D) 3-cyanochroman-2-one product. The cyano groups were ionized by in-source fragmentation. LC/MS spectra of the dinitrophenylhydrazone derivatives of the (E) 2-cyclohexen-1-one standard and (F) cyclohexanone product. A mass spectrum of the underivatized product could not be obtained. In all cases, corresponding compounds are shown to the right of the spectra. A mass spectrum showing the reduction of malachite green to leucomalachite green was previously published (Jirapanjawat et al., 2016).
