Fig. 2.
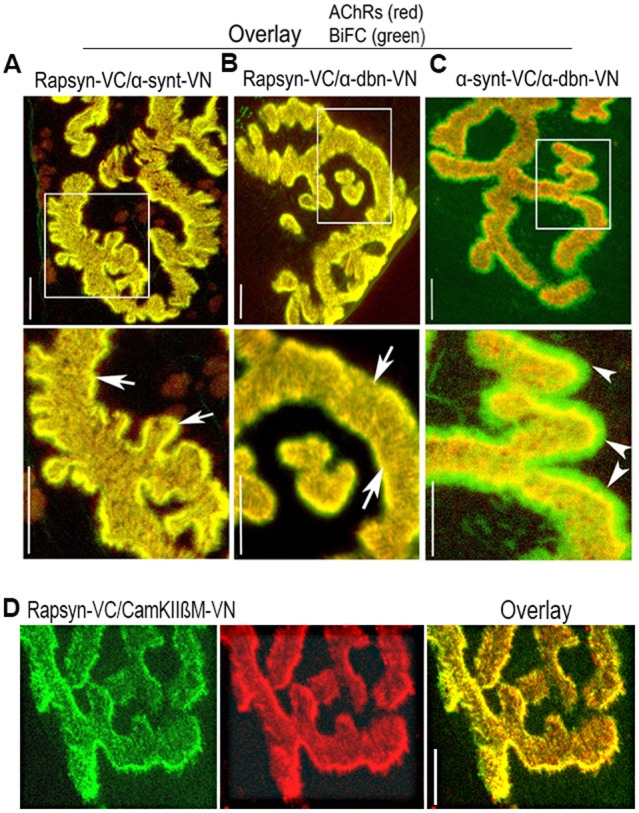
In vivo BiFC shows interaction between rapsyn, α-dystrobrevin, α-syntrophin and CamKIIβM at the NMJ. Sternomastoid muscles were co-electroporated either with rapsyn–VC and α-syntrophin–VN (α-synt–VN), or rapsyn–VC and α-dystrobrevin–VN (α-dbn–VN), or α-dystrobrevin–VN and α-syntrophin–VC (α-synt–VC); 7 days later, muscles were bathed with BTX–Alexa594 to label AChRs, and synapses expressing BiFC signals were imaged. (A) A representative synapse showing that the fluorescence complementation between rapsyn–VC and α-syntrophin–VN is restricted to the crests of junctional folds where they precisely colocalized with AChRs (see arrows). (B) A representative synapse image showing fluorescence complementation between rapsyn–VC and α-dystrobrevin–VN (α-dbn–VN) only at the crests of junctional folds that were identified by AChR labeling (see arrows). (C) A representative synapse image showing the fluorescence complementation between α-syntrophin and α-dystrobrevin at both the crest and troughs of junctional folds (see arrowheads). Boxed areas (above) are enlarged in the images below. (D) A representative image showing the BiFC signal between rapsyn–VC and CamKIIβM–VN. Scale bars: 5 µm. Eight mice were used in each experiment.
