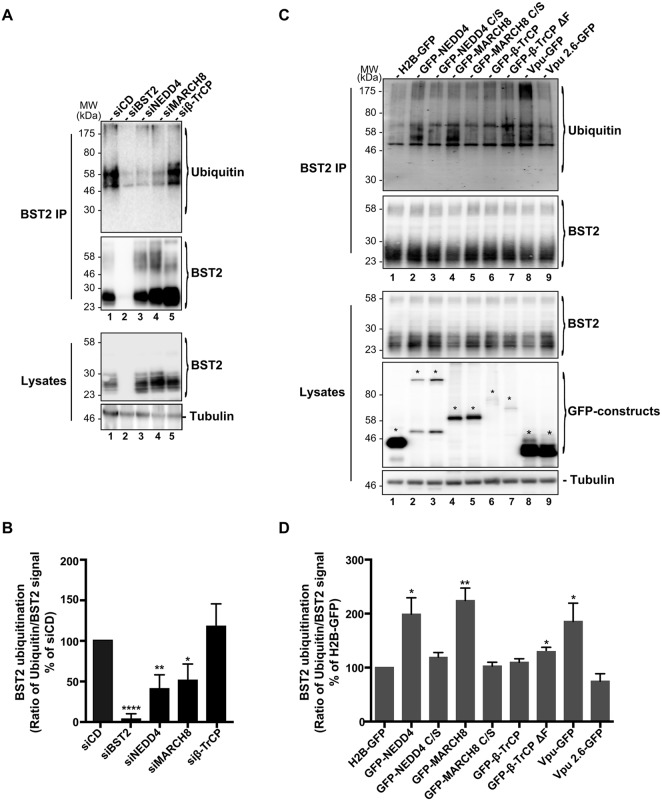
Fig. 2.
Contribution of NEDD4, MARCH8 and β-TrCP to BST2 ubiquitylation. (A,B) siRNA-transfected HeLa cells or (C,D) HeLa cells transfected with plasmid encoding wild-type (lanes 2, 4, 6) or catalytically inactive mutants (lanes 3, 5, 7) of NEDD4, MARCH8 or β-TrCP fused to GFP were lysed in stringent buffer, and BST2 was immunoprecipitated (IP) with an antibody against BST2. Ubiquitylation of BST2 was analyzed by western blot using an antibody against ubiquitin. In panels C,D, plasmids encoding GFP-tagged histone H2B (H2B–GFP), or WT Vpu (Vpu–GFP) or Vpu mutated on residues S52 and S56 (Vpu2.6–GFP) were used as controls. Asterisks in panel C indicate the bands corresponding to GFP fusion proteins. (B,D) Signals obtained for ubiquitin staining were normalized to those obtained for BST2. Values obtained for each condition were normalized to those obtained for control cells (siCD and H2B–GFP, respectively) set as 100%. Bars represent the mean±s.e.m. (n=4), ****P<0.0001, **P<0.01, *P<0.05.