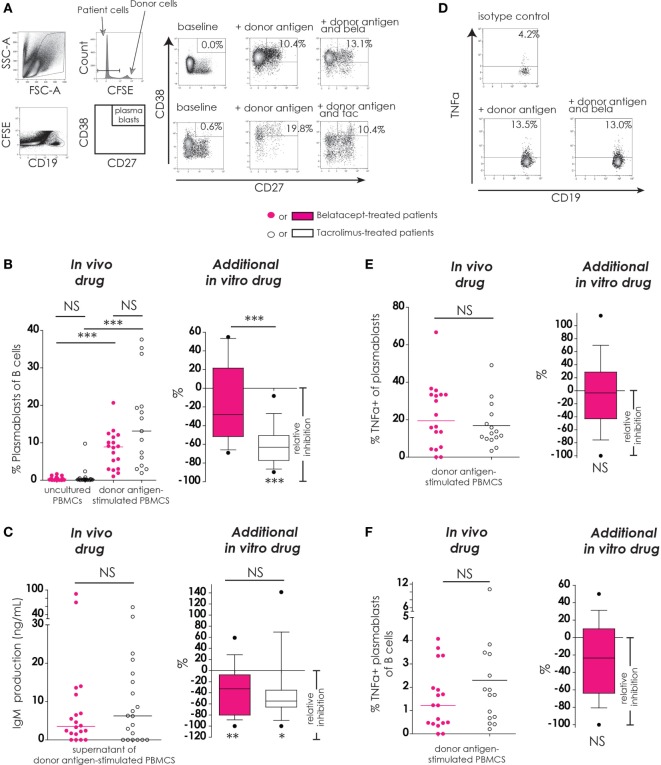
Figure 5.
Belatacept in vitro did not inhibit donor antigen-driven plasmablast formation or TNFα production in a peripheral blood mononuclear cell (PBMC)-based assay, but suppressed IgM production. The gating strategy is depicted for plasmablasts (CD19+CD27+CD38++) after 7 days of donor antigen stimulation, in the presence or absence of belatacept and tacrolimus (A). Donor PBMCs were discriminated by carboxyfluorescein succinimidyl ester labeling them prior to the mixed lymphocyte reaction and gating them out after. The proportions of plasmablasts are shown for 7-day donor antigen-stimulated PBMCs obtained from the belatacept or tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept or tacrolimus (“additional in vitro” column) (B). The IgM concentrations in the supernatants are shown for the same cultures as previously mentioned (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept or tacrolimus (“additional in vitro” column) (C). A typical example is depicted for intracellular TNFα-production by plasmablasts after 7 days of donor antigen stimulation, in the presence or absence of belatacept (D). The proportions of TNFα+ cells within plasmablasts (E) and the proportions of TNFα+ plasmablasts within B-cells (F) are shown for 7-day donor antigen-stimulated PBMCs obtained from the belatacept or tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept (“additional in vitro” column). The proportions of TNFα+ plasmablasts could not be reliably determined in the presence of tacrolimus in vitro, because of the strong inhibition of plasmablast formation by tacrolimus. In the graphs in the “In vivo drug” columns, every dot represents PBMCs of a single patient. In the graphs in the “additional in vitro drug” columns, the relative inhibitions by additional in vitro belatacept and tacrolimus are depicted for aforementioned cell populations in the same belatacept- and tacrolimus-treated patients. The proportions of these cell populations after donor antigen stimulation in the absence of in vitro drugs are set to 0. The median relative inhibitions by belatacept and tacrolimus were tested against a theoretical median of 0. Asterisks below boxes depict the p-values of these tests. The relative inhibitions were compared between in vitro belatacept and tacrolimus. Lines in boxes represent medians, borders of boxes represent 25th and 75th percentiles, and error bars present 10th and 90th percentiles. Every box represents cultures of PBMCs obtained from n = 20 belatacept-treated or n = 20 tacrolimus-treated patients. *p < 0.05, **p < 0.01, ***p < 0.001, NS, not significant. N.B.: The median fluorescence intensity slightly decreases in (antigen-) stimulated cells compared to unstimulated cells, partly because of the intracellular staining protocol that was used to determine intracellular cytokine expression. Therefore, the gates in the unstimulated and stimulated cells are not exactly the same.