Abstract
Three new indolediterpenoids, namely, 22-hydroxylshearinine F (1), 6-hydroxylpaspalinine (2), and 7-O-acetylemindole SB (3), along with eight related known analogs (4–11), were isolated from the sea-anemone-derived fungus Penicillium sp. AS-79. The structures and relative configurations of these compounds were determined by a detailed interpretation of the spectroscopic data, and their absolute configurations were determined by ECD calculations (1 and 2) and single-crystal X-ray diffraction (3). Some of these compounds exhibited prominent activity against aquatic and human pathogenic microbes.
Keywords: endophytic fungus, Penicillium sp., secondary metabolites, antimicrobial activity
1. Introduction
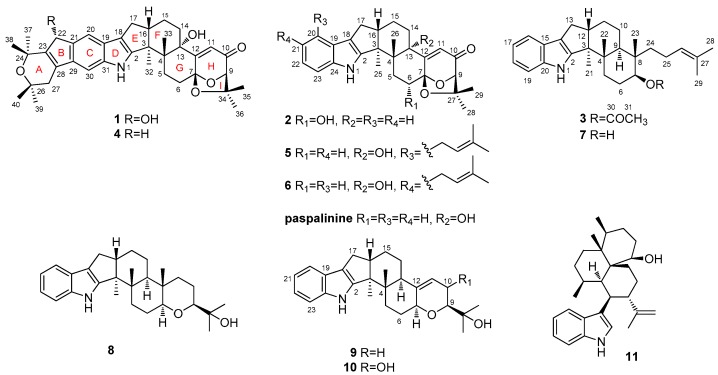
Indolediterpenoids (IDTs) are a group of fungal metabolites that have attracted the attention of natural product chemists due to their diversified structures and potent biological activities [1,2]. IDTs usually possess a common core structure comprised of an indole moiety and is most likely derived from indole-3-glycerol phosphate and a geranyldiphosphate-derived cyclic diterpenoid skeleton [3]. The typical IDTs reported from fungal sources are penitrems, janthitrems, lotitrems, aflatrems, paxilline, and shearinines [2]. Previous reports revealed that IDTs possessed various bioactivities including antibiotic [4,5] and anti-insectan potency [6,7] as well as tremorgenic mammalian mycotoxicity [8,9]. During our investigation on marine-derived fungi, a series of structurally interesting secondary metabolites such as cyclohexadepsipeptides [10], diketopiperazine alkaloids [11], and diphenylketones [12] were discovered. Recently, we focused our attention on Penicillium sp. AS-79, a fungal strain isolated from the fresh tissue of the sea anemone Haliplanella luciae, and, as a result, three new (1–3) and eight known (4–11) indolediterpenoids are isolated and elucidated (Figure 1). Details of the structure determination and biological activities of these compounds are presented herein.
Figure 1.
Structures of 1–11 and paspalinine.
2. Results and Discussion
2.1. Structure Elucidation of the New Compounds
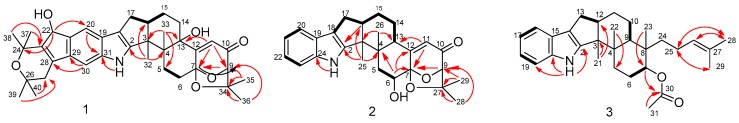
Compound 1 was obtained as a colorless solid. Its molecular formula was determined as C37H45NO6 on the basis of (+)-HRESIMS data, with 16 degrees of unsaturation. The 1H NMR data (Table 1) revealed the presence of eight methyl singlets and six methines (including three aromatic/olefinic and two oxygenated) as well as six methylenes in 1. Its 13C NMR and DEPT data (Table 1) showed the presence of 37 carbon resonances, including eight methyls, six sp3 methylenes, six methines (including three aromatic/olefinic, two oxygenated and/or heteroatom-bonded, and one sp3 methine), and 17 quaternary carbons. The 1H and 13C data of 1 are very similar to those of shearinine F (4), an indolediterpenoid isolated from the marine mangrove-derived endophytic fungus Penicillium sp. (strain HKI0459) [13], indicating that 1 is also an indolediterpenoid derivative. Detailed comparison of the NMR data between 1 and 4 revealed that the methylene group at δC 36.2/δH 3.38 (CH2-22) of 4 was replaced by an oxygenated methine group at δC 76.3/δH 5.19 (CH-22) (Table 1). The observed HMBC correlations from H-22 to C-20 and C-24 confirmed the replacement of C-22 methylene in 4 by oxymethine in 1 (Figure 2).
Table 1.
1H (125 MHz) and 13C NMR (500 MHz) data of Compounds 1–3 (δ in ppm, J in Hz).
| No. | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 7.75, s | 7.73, s | 9.79, s | |||
| 2 | 151.8, C | 149.9, C | 151.8, C | |||
| 3 | 51.8, C | 51.2, C | 53.9, C | |||
| 4 | 40.1, C | 38.1, C | 38.6, C | |||
| 5 | α 2.79, m | 27.2, CH2 | α 2.59, d (14.8) | 37.5, CH2 | α 1.80, m | 24.7, CH2 |
| β 1.72, m | β 2.17, d (5.2) | β 1.73, m | ||||
| 6 | α 2.82, m | 28.4, CH2 | 4.15, d (5.0) | 70.4, CH | α 1.89, m | 23.3, CH2 |
| β 2.05, m | β 1.69, m | |||||
| 7 | 104.5, C | 103.3, C | 4.88, dd (10.7, 4.3) | 75.9, CH | ||
| 8 | 40.0, C | |||||
| 9 | 4.33, s | 88.2, CH | 4.37, s | 88.4, CH | 1.51, br.s | 41.0, CH |
| 10 | 197.2, C | 195.2, C | α 1.79, m | 28.0, CH2 | ||
| β1.72, m | ||||||
| 11 | 5.86, s | 117.8, CH | 5.87, s | 118.7, CH | α 1.80, m | 33.1, CH2 |
| β 1.68, m | ||||||
| 12 | 169.8, C | 171.3, C | 2.80, m | 49.8, CH | ||
| 13 | 77.8, C | 3.68, dt (3.4, 11.7) | 39.5, CH | α 2.36, dd (13.1, 10.9) | 28.0, CH2 | |
| β 2.68, dd (13.1, 6.4) | ||||||
| 14 | α 2.05, m | 34.5, CH2 | α 1.97, m | 24.1, CH2 | 117.9, C | |
| β 1.92, m | β 1.48, m | |||||
| 15 | α 2.05, m | 21.3, CH2 | α 1.85, m | 24.0, CH2 | 131.5, C | |
| β 1.72, m | β 1.77, m | |||||
| 16 | 2.64, m | 48.6, CH | 2.83, m | 48.4, CH | 7.36, d (7.4) | 118.8, CH |
| 17 | α 2.79, dd (12.4, 5.6) | 27.8, CH2 | α 2.74, dd (13.2, 6.3) | 27.8, CH2 | 6.99, dt (7.1, 1.6) | 119.7, CH |
| β 2.05, dd (12.4, 3.1) | β 2.41, dd (13.2, 10.6) | |||||
| 18 | 117.8, C | 118.5. C | 6.99, dt (7.1, 1.6) | 120.6, CH | ||
| 19 | 123.6, C | 125.3, C | 7.32, d (7.3) | 112.6, C | ||
| 20 | 7.56, s | 114.1, CH | 7.46, d (7.9) | 119.9, CH | 7.32, d (7.3) | 141.7, C |
| 21 | 136.8, C | 7.11, t (6.2) | 120.8, CH | 1.10, s | 15.0, CH3 | |
| 22 | 5.19, s | 76.3, CH | 7.11, t (6.2) | 121.5, CH | 1.18, s | 19.4, CH3 |
| 23 | 144.8, C | 7.34, d (8.2) | 111.7, CH | 0.95, s | 18.1, CH3 | |
| 24 | 73.8, C | 140.3, C | α 1.52, m | 29.9, CH2 | ||
| β 1.23, m | ||||||
| 25 | 1.16, s | 15.5, CH3 | α 2.08, m | 22.1, CH2 | ||
| β 1.80, m | ||||||
| 26 | 71.3, C | 1.18, s | 21.6, CH3 | 5.11, t (7.1) | 125.5, CH | |
| 27 | α 2.45, d (18.0) | 34.1, CH2 | 79.8, C | 126.1, C | ||
| β 2.43, d (18.0) | ||||||
| 28 | 138.4, C | 1.44, s | 28.7, CH3 | 1.63, s | 25.9, CH3 | |
| 29 | 133.9, C | 1.24, s | 23.2, CH3 | 1.69, s | 17.6, CH3 | |
| 30 | 7.02, s | 102.1, CH | 170.6, C | |||
| 31 | 140.0, C | 2.05, s | 21.1, CH3 | |||
| 32 | 1.37, s | 16.3, CH3 | ||||
| 33 | 1.25, s | 23.8, CH3 | ||||
| 34 | 78.9, C | |||||
| 35 | 1.20, s | 23.2, CH3 | ||||
| 36 | 1.46, s | 29.0, CH3 | ||||
| 37 | 1.55, s | 30.4, CH3 | ||||
| 38 | 1.50, s | 30.0, CH3 | ||||
| 39 | 1.29, s | 29.7, CH3 | ||||
| 40 | 1.37, s | 29.5, CH3 | ||||
a Measured in CDCl3. b Measured in acetone-d6.
Figure 2.
Key COSY (bold line) and HMBC (arrow line) correlations of Compounds 1–3.
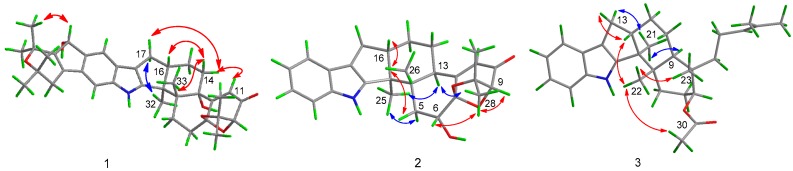
The relative configuration of 1 was determined by the NOESY spectrum. The NOESY correlations from H-32 to H-17α, from H-14β to H-16 and H-33, and from H-14α to H-11 confirmed the trans fusion of Rings E and F and of Rings F and G. For Rings A and B, the NOESY correlation between H-22 and H-38 placed the OH group on C-22 and CH3-38 on the opposite face (Figure 3).
Figure 3.
NOESY correlations of Compounds 1–3.
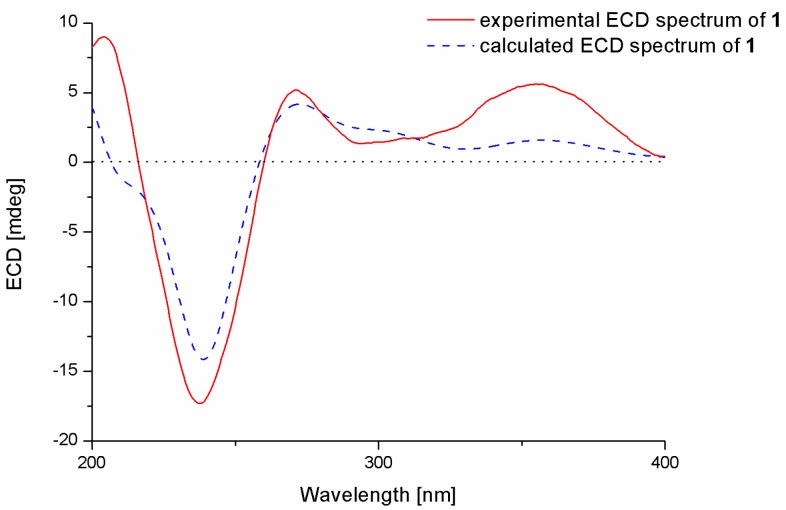
To determine the absolute configurations of the stereogenic carbons and of the compound, conformational search, geometry optimization of each possible isomer, and time-dependent density functional (TDDFT)-ECD calculation were performed using Gaussian 09.10. The obtained minimum energy conformers were calculated for their ECD spectra by the TDDFT method at the B3LYP/6-31G(d) level. The experimental ECD spectrum of 1 matched well with that calculated for 3S, 4R, 7S, 9R, 13S, 16S, and 22R configurations, which showed positive cotton effects (CEs) at approximately 355 and 272 nm and a negative CE near 237 nm (Figure 4). Thus, the structure of 1 was determined and was named 22-hydroxylshearinine F.
Figure 4.
Experimental and calculated ECD spectra of 1.
Compound 2 was obtained as a colorless solid. Its molecular formula was determined as C27H31NO4 on the basis of (+)-HRESIMS, indicating 13 degrees of unsaturation. The 1H and 13C NMR spectra of 2 are very similar to those of paspalinine (Figure 1), an indolediterpenoid isolated from the marine mangrove-derived endophytic fungus Alternariatenuissima EN-192 [14]. However, inspection of the NMR data revealed that CH2-6 (δC 29.5; δH 2.41/2.64) in paspaline was replaced by an oxymethine group (δC 70.4; δH 4.15) (Table 1). This was further supported by the observed COSY correlation between H-5 and H-6 as well as by the HMBC correlations from H-6 to C-4, C-7, and C-12 (Figure 2).
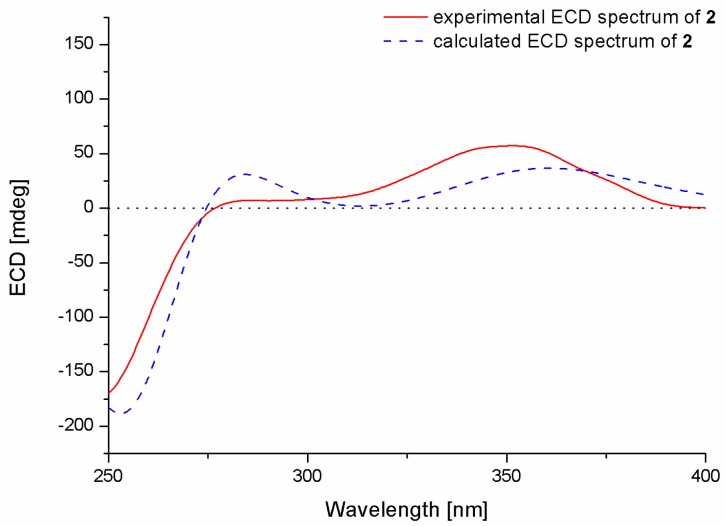
The relative configurations of the stereogenic carbons of 2 were determined by NOESY spectrum. The NOESY correlations from H-26 to H-5β and H-16 indicated the same orientation of these protons, while the correlations from H-25 to H-5α and H-13 and from H-13 to H-28 as well as from H-28 to H-6 and H-9 indicated the opposite orientation of these groups (Figure 3). The absolute configurations of the stereogenic carbons of 2 were also determined by the TDDFT-ECD calculation in Gaussian 09.10. The experimental ECD spectrum of 2 showed excellent agreement with that calculated for 3S, 4S, 6R, 7R, 9R, 13R, and 16S configurations (Figure 5), and the structure of 2 was thus established and was named 6-hydroxylpaspalinine.
Figure 5.
Experimental and calculated ECD spectra of 2.
Compound 3 was obtained as colorless crystals. Its molecular formula was determined as C30H41NO2 on the basis of (+)-HRESIMS data, indicating 11 degrees of unsaturation. Except for the presence of the acetyl group (δC 170.6; δC 21.1/δH 2.05, s), the NMR data of 3 are very similar to those of emindole SB (7), an indolediterpenoid isolated from the marine mangrove-derived endophytic fungus Dichotomomyces cejpii [15], indicating that 3 is an acetylated derivative of 7.
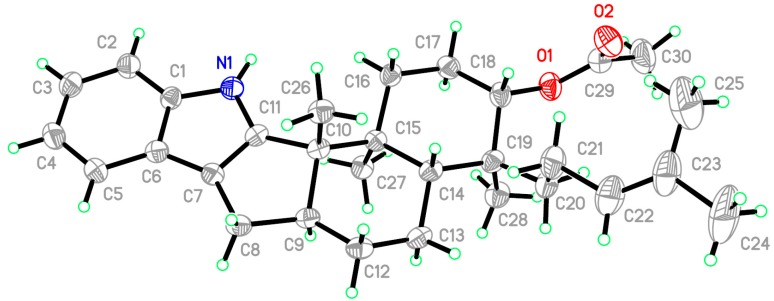
The relative configurations of the stereogenic carbons of 3 were determined by NOESY spectrum. The NOESY correlations from H-21 to H-9 and H-13α indicated the same orientation of these protons, while correlations from H-22 to H-12, H-23, and H-31 as well as from H-12 to H-13β indicated the other orientation of these protons (Figure 3). X-ray analysis confirmed the structure of 3 and determined the absolute configurations of C-3, 4, 7, 8, and 9 as 3S, 4S, 7S, 8S, and 9S. The ortep view of 3 is shown in Figure 6. Compound 3 was named 7-O-acetylemindole SB.
Figure 6.
X-ray structure of Compound 3 (note: a different numbering system is used for the structure in the text).
In addition to the isolation of Compounds 1–3, eight other congeners—shearinine F (4) [13], paspalitrem C (5) [16], paspalitrem A (6) [16], emindole SB (7) [15], paspaline (8) [17], 3-deoxo-4b-deoxypaxilline (9) [18], PC-M6 (10) [19], and 10,23-dihydro-24,25-dehydroaflavinine (11) [20]—were also isolated and identified. Their structures were determined by analysis of the spectroscopic data and by comparison with that reported in the literature.
2.2. Biological Activities of the Isolated Compounds
The isolated compounds (1–11) were examined for antimicrobial activity against several human-, aqua-, and plant-pathogenic microbes. Compounds 5, 7, and 11 showed activity against the aquatic pathogen Pseudomonas aeruginosa, with MIC values of 8.0, 1.0, and 0.5 μg/mL, respectively, which are comparable to that of the positive control, chloromycetin (MIC = 0.5 μg/mL). Moreover, Compounds 5, 7, 8, and 11 exhibited activity against the human pathogen Escherichia coli with MIC values of 16.0, 4.0, 0.5, and 2.0 μg/mL, respectively, while the positive control chloromycetin has an MIC value of 1.0 μg/mL. Compounds 2, 5, 7, 9, and 11 exhibited activity against the aquatic pathogen Vibrio parahaemolyticus with MIC values of 64.0, 8.0, 2.0, 16.0, and 0.5 μg/mL, respectively, while the positive control chloromycetin has an MIC value of 0.5 μg/mL. Compounds 5, 7, and 11 exhibited activity against V. alginolyticus with MIC values of 4.0, 1.0, and 0.5 μg/mL, respectively, while the positive control chloromycetin has an MIC value of 0.5 μg/mL.
These data indicated that Compound 5, with a prenyl group at C-20, has stronger antimicrobial activity than does Compound 6, where the prenyl substituent is at C-21, while the results from Compounds 8, 9, and 10 showed that the double bond at C-11 and the hydroxyl substitute at C-10 significantly influenced the activity. Moreover, acetylation of OH at C-7 decreased the antibacterial activity (3 vs. 7).
3. Experimental Section
3.1. General
Melting points were determined with an SGW X-4 micro-melting-point apparatus. Optical rotations were measured on an Optical Activity AA-55 polarimeter (Optical Activity Ltd., Cambridgeshire, UK). UV spectra were measured on a Lengguang Gold S54 spectrophotometer (Shanghai Lengguang Technology Co. Ltd., Shanghai, China). ECD spectra were acquired on a JASCO J-715 spectropolarimeter (JASCO, Tokyo, Japan). The 1H, 13C, and 2D NMR spectra were acquired using Bruker Avance 500 spectrometers (Bruker Biospin Group, Karlsruhe, Germany). Mass spectra were determined on a VG Autospec 3000 (VG Instruments, London, UK) or an API QSTAR Pulsar 1 mass spectrometer (Applied Biosystems, Foster, Waltham, MA, USA). Analytical HPLC and semi-preparative HPLC were performed using a Dionex HPLC system equipped with a P680 pump, an ASI-100 automated sample injector, and a UVD340U multiple wavelength detector controlled by Chromeleon software (version 6.80) (Dionex, Sunnyvale, CA, USA). Column chromatography (CC) was performed with silica gel (200–300 mesh, Qingdao Haiyang Chemical Factory, Qingdao, China), Lobar LiChroprep RP-18 (40–60 μm, Merck, Darmstadt, Germany), and Sephadex LH-20 (18–110 μm, Merck).
3.2. Fungal Material
The fungus Penicillium sp. AS-79 was isolated from the fresh tissue of the sea anemone Haliplanella luciae, which was collected from the Qingdao coastline in December 2014. The fungus was identified by sequence analysis of the ITS [21] and beta-tubulin [22] region of its rDNA as described previously. The resulting sequence data obtained from the fungal strain have been deposited in GenBank (with accession No. KY322516). A BLAST search result indicated that the ITS sequence was most similar (99%) to the sequence of Penicillium janthinellum (compared to KM268705.1), but the beta-tubulin sequence was most similar (95%) to P. vasconiae (compared to KT887805.1). Therefore, it is hard to identify the strain to a species level. The strain is preserved at the key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences.
3.3. Fermentation
The fermentation was carried out statically on a rice solid medium (each flask contained 70 g of rice (Cofco, Beijing, China), 0.1 g of corn flour (Macklin, Shanghai, China), 0.3 g of peptone (Shuangxuan, Beijing, China), 0.1 g of sodium glutamate (Lianhua, Henan, China), and 100 mL of naturally sourced and filtered seawater, which was obtained from the Huiquan Gulf of the Yellow Sea near the campus of the authors’ institution, pH 6.5–7.0) in 1 L Erlenmeyer flasks for 30 days at room temperature.
3.4. Extraction and Isolation
The whole fermented broth (66 flasks) was extracted exhaustively with EtOAc, yielding 72.5 g of crude extract, which was subjected to silica gel vacuum liquid chromatography (VLC) eluting with mixed solvents of increasing polarity (petroleum ether (PE) to MeOH) to yield nine fractions (Fraction 1 to Fraction 9). Fraction 3 (5.1 g) was further purified by reverse-phase column chromatography (CC) over Lobar LiChroprep RP-18 with a MeOH–H2O gradient (from 20:80 to 100:0) to afford two subfractions (Fraction 3.1 and Fraction 3.2). Fraction 3.2 was further purified by prep. TLC (plate: 20 × 20 cm, developing solvents: PE/acetone, 10:1), then by CC on Sephadex LH-20 (MeOH), and finally by prep. TLC (plate: 20 × 20 cm, developing solvents: CH2Cl2/MeOH, 40:1) to obtain Compounds 3 (71.8 mg) and 11 (12.4 mg). Fraction 4 (2.0 g) was further purified by CC on silica gel (eluted with CH2Cl2-MeOH, 200:1 to 60:1), then on Sephadex LH-20 (MeOH) and finally on silica gel (eluted with CH2Cl2) to obtain Compound 6 (11.8 mg). Fraction 5 (5.9 g) was further purified by CC over Lobar LiChroprep RP-18 with a MeOH–H2O gradient (from 20:80 to 100:0) to afford eight subfractions (Fraction 5.1–Fraction 5.8). Fraction 5.4 and Fraction 5.5 was further purified by CC on silica gel (eluted with CH2Cl2-MeOH, 100:1 to 60:1). Then Fraction 5.4 was purified by prep. TLC (plate: 20 × 20 cm, developing solvents: CH2Cl2/MeOH, 40:1) to obtain Compound 1 (12.3 mg). Fraction 5.5 (3.5 g) was further purified by CC on Sephadex LH-20 (MeOH) to obtain Compound 2 (5.2 mg). Fraction 5.7 (48.9 mg) was further purified by CC on silica gel (eluted with CH2Cl2-MeOH, 200:1 to 100:1) to obtain Compounds 4 (5.8 mg) and 8 (8.9 mg). Fraction 5.8 (139 mg) was further purified by CC on silica gel (eluted with CH2Cl2-MeOH, 200:1 to 100:1) and by prep. TLC (plate: 20 × 20 cm, developing solvents: CH2Cl2/MeOH, 80:1) to obtain Compounds 5 (10.0 mg) and 7 (9.7 mg). Fraction 6 (5.8 g) was further purified by CC over Lobar LiChroprep RP-18 with a MeOH–H2O gradient (from 20:80 to 100:0) to afford five subfractions (Fraction 6.1–Fraction 6.5). Fraction 6.4 (100.0 mg) was further purified by CC on silica gel (eluted with PE-EtOAc, 10:1 to 8:1) to obtain Compound 9 (4.9 mg). Fraction 6.5 was further purified by prep. HPLC (MeOH–H2O, 85:15) to obtain Compound 10 (11.2 mg, tR 13.0 min).
22-Hydroxylshearinine F (1): colorless solid; : +50 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 215 (4.39), 251 (4.29), 317 (4.02) nm; ECD λmax (Δε) 237.5 (−12.18), 272 (+3.54) nm, 1H and 13C NMR data, see Table 1; ESIMS m/z 600 [M + H]+; HRESIMS m/z 600.3330 [M + H]+ (calcd. for C37H46NO6, 600.3320).
6-Hydroxylpaspalinine (2): yellowish solid; : +30.8 (c 0.13, MeOH); UV (MeOH) λmax (log ε) 221 (3.51), 232 (3.49) nm; ECD λmax (Δε) 250 (−9.79), 354.5 (+30.62) nm;1H and 13C NMR data, see Table 1; ESIMS m/z 434 [M + H]+; HRESIMS m/z 434.2332 [M + H]+ (calcd. for C27H32NO4, 434.2326).
7-O-Acetylemindole SB (3): colorless crystals; m.p. 192–194 °C; : +45.5 (c 0.11, MeOH); UV (MeOH) λmax (log ε) 229 (3.80), 281 (3.17) nm; ECD λmax (Δε) 250 (+6.50), 295 (−2.79) nm; 1H and 13C NMR data, see Table 1; ESIMS m/z 448 [M + H]+; HRESIMS m/z 448.3207 [M + H]+ (calcd. for C30H42NO2, 448.3210).
3.5. X-ray Crystallographic Analysis of Compound 3
All crystallographic data were collected on an Agilent Xcalibur Eos Gemini CCD plate diffractometer (Agilent Technologies, Santa Clara, CA, USA), using graphite monochromatized Cu Kα radiation (λ = 1.54178 Å) for 3 [23]. The data were corrected for absorption by using the program SADABS [24]. The structures were solved by direct methods with the SHELXTL software package (Sheldrick G.M., University of Göttingen, Germany) [25]. All non-hydrogen atoms were refined anisotropically. The H atoms were located by geometrical calculations, and their positions and thermal parameters were fixed during the structure refinement. The structure was refined by full-matrix least-squares techniques [26].
Crystal data for compound 3: C30H41NO2, F.W. = 447.64, orthorhombic space group, P2(1)2(1)2(1), unit cell dimensions a = 6.8164(4) Å, b = 7.6058(6) Å, c = 50.959(3) Å, V = 2641.9(3) Å3, α = β = γ = 90°, Z = 4, dcalcd = 1.125 mg/m3, crystal dimensions 0.40 × 0.33 × 0.14 mm, μ = 0.531 mm−1, F(000) = 976. The 4587 measurements yielded 306 independent reflections after equivalent data were averaged, and Lorentz and polarization corrections were applied. The final refinement gave R1 = 0.0641 and wR2 = 0.1236 [I > 2σ(I)]. The final refinement Flack parameter is 0.0(5).
3.6. Antimicrobial Assay
Antimicrobial assay against human- and aqua-pathogenic microbes Edwardsiella tarda, Escherichia coli, Micrococcus luteus, Pseudomonas aeruginosa, Vibrio alginolyticus, V. harveyi, V. parahaemolyticus, and plant pathogenic fungi Alternaria alternata, A. brassicae, Colletotrichum gloeosprioides, Fusarium graminearum, F. oxysporum, Gaeumannomyces graminis, Phytophthora nicotiana, Physalospora piricola, and Valsa mali was carried out using the well diffusion method [27]. Chloromycetin was used as a positive control for the bacteria, while amphotericin B was used as a positive control for the fungi.
4. Conclusions
Three new indolediterpenoid derivatives (1–3), along with eight known analogs (4–11), were isolated from the sea anemone-derived fungus Penicillium sp. AS-79. The structures and relative configurations were determined via the interpretation of NMR data, and the absolute configurations of all compounds were determined via ECD comparison, while the structure of Compound 3 was confirmed by single-crystal X-ray diffraction analysis. Several compounds exhibited activity against some of the tested microbial strains.
Acknowledgments
This work was financial supported by the NSFC-Shandong Joint Fund for Marine Science Research Centers (U1406402) and by the Scientific and Technological Innovation Project of Qingdao National Laboratory for Marine Science and Technology (No. 2015ASKJ02). B.-G.W. acknowledges the support of Taishan Scholar Program from Shandong Province.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/5/137/s1. 1D and 2D NMR spectra of Compounds 1–3 as well as crystal packing of Compound 3.
Author Contributions
X.-Y.H. performed the experiments of isolation, structure elucidation, and antimicrobial evaluation, and prepared the manuscript; L.-H.M. contributed to part of the structure determination; X.L. performed the ECD calculations, S.-Q.Y. isolated the fungus from sea-anemone; X.-M.L. performed the 1D and 2D NMR experiments, B.-G.W. supervised the research work and revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sun K.L., Li Y., Guo L., Wang Y., Liu P.P., Zhu W.M. Indolediterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saikia S., Nicholson M.J., Young C., Parker E.J., Scott B. The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol. Res. 2008;112:184–199. doi: 10.1016/j.mycres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Byrne K.M., Smith S.K., Ondeyka J.G. Biosynthesis of nodulisporic acid A: Precursor studies. J. Am. Chem. Soc. 2002;124:7055–7060. doi: 10.1021/ja017183p. [DOI] [PubMed] [Google Scholar]
- 4.Qiao M.F., Ji N.Y., Liu X.H., Li K., Zhu Q.M., Xue Q.Z. Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorg. Med. Chem. Lett. 2010;20:5677–5680. doi: 10.1016/j.bmcl.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Ding L., Maier A., Fiebig H.H., Lin W.H., Hertweck C. A family of multicyclic indolosesquiterpenes from a bacterial endophyte. Org. Biomol. Chem. 2011;9:4029–4031. doi: 10.1039/c1ob05283g. [DOI] [PubMed] [Google Scholar]
- 6.Hensens O.D., Ondeyka J.G., Dombrowski A.W., Ostlind D.A., Zink D.L. Isolation and structure of nodulisporic acid A1 and A2, novel insecticides from a Nodulisporium sp. Tetrahedron Lett. 1999;40:5455–5458. doi: 10.1016/S0040-4039(99)01064-3. [DOI] [Google Scholar]
- 7.Knaus H.G., McManus O.B., Lee S.H., Schmalhofer W.A., Garcia-Calvo M., Helms L.M.H., Sanchez M., Giangiacomo K., Reuben J.P., Smith A.B., III, et al. Tremorgenicindole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 8.Li C., Gloer J.B., Wicklow D.T., Dowd P.F. Thiersinines A and B: Novel antiinsectan indole diterpenoids from a new fungicolous Penicillium species (NRRL 28147) Org. Lett. 2002;4:3095–3098. doi: 10.1021/ol026424a. [DOI] [PubMed] [Google Scholar]
- 9.Ondeyka J.G., Helms G.L., Hensens O.D., Goetz M.A., Zink D.L., Tsipouras A., Shoop W.L., Slayton L., Dombrowski A.W., Polishook J.D., et al. Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp. isolation, structure determination, and chemical transformations. J. Am. Chem. Soc. 1997;119:8809–8816. doi: 10.1021/ja971664k. [DOI] [Google Scholar]
- 10.Du F.Y., Zhang P., Li X.M., Li C.C., Cui C.M., Wang B.G. Cyclohexadepsipeptides of the isaridin class from the marine-derived fungus Beauveriafelina EN-135. J. Nat. Prod. 2014;77:1164–1169. doi: 10.1021/np4011037. [DOI] [PubMed] [Google Scholar]
- 11.Meng L.H., Wang C.Y., Mándi A., Li X.M., Hu X.Y., Kassack U.M., Kurtán T., Wang B.G. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endophytic fungus Penicillium brocae MA-231. Org. Lett. 2016;18:5304–5307. doi: 10.1021/acs.orglett.6b02620. [DOI] [PubMed] [Google Scholar]
- 12.Li H.L., Li X.M., Liu H., Meng L.H., Wang B.G. Two new diphenylketones and a new xanthone from Talaromyces islandicus EN-501, an endophytic fungus derived from the marine red alga Laurencia okamurai. Mar. Drugs. 2016;14:223. doi: 10.3390/md14120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M.J., Gessner G., Groth I., Lange C., Christner A., Bruhn T., Deng Z.W., Li X., Heinemann S.H., Grabley S., et al. Shearinines D-K, new indoletriterpenoids from an endophytic Penicillium sp. (strain HKI0459) with blocking activity on large-conductance calcium-activated potassium channels. Tetrahedron. 2007;63:435–444. doi: 10.1016/j.tet.2006.10.050. [DOI] [Google Scholar]
- 14.Sun H., Gao S.S., Li X.M., Li C.S., Wang B.G. Chemical constituents of marine mangrove-derived endophytic fungus Alternaria tenuissima EN-192. Chin. J. Oceanol. Limnol. 2013;31:464–470. doi: 10.1007/s00343-013-2106-2. [DOI] [Google Scholar]
- 15.Harms H., Rempel V., Kehraus S., Kaiser M., Hufendiek P., Müller C.E., König G.M. Indoloditerpenes from a marine-derived fungal strain of Dichotomomyces cejpii with antagonistic activity at GPR18 and cannabinoid receptor. J. Nat. Prod. 2014;77:673–677. doi: 10.1021/np400850g. [DOI] [PubMed] [Google Scholar]
- 16.Dorner W.J., Cole J.R., Cox H.R., Cunfer M.B. Paspalitrem C, a new metabolite from sclerotia of Claviceps paspali. J. Agric. Food Chem. 1984;32:1069–1071. doi: 10.1021/jf00125a033. [DOI] [PubMed] [Google Scholar]
- 17.Springer P.J., Clardy J., Wells M.J., Cole J.R., Kirksey W. The structure of paxilline, a tremorgenic metabolite of Penicillium paxilli bainier. Tetrahedron Lett. 1975;16:2531–2534. doi: 10.1016/S0040-4039(00)75170-7. [DOI] [Google Scholar]
- 18.Fan Y.Q., Wang Y., Liu P.P., Fu P., Zhu T.H., Wang W., Zhu W.M. Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J. Nat. Prod. 2013;76:1328–1337. doi: 10.1021/np400304q. [DOI] [PubMed] [Google Scholar]
- 19.Hosoe T., Nozawa K., Udagawa S.I., Nakajima S., Kawai K.I. Structures of new indoloditerpenes, possible biosynthetic precursors of the tremorgenic mycotoxins, penitrems, from Penicillium crustosum. Chem. Pharm. Bull. 1990;38:3473–3475. doi: 10.1248/cpb.38.3473. [DOI] [Google Scholar]
- 20.Tepaske R.M., Gloer B.J., Wicklow T.D., Dowd F.P. Three new aflavinines from sclerotia of Aspergillus tubingensis. Tetrahedron. 1989;45:4961–4968. doi: 10.1016/S0040-4020(01)81077-2. [DOI] [Google Scholar]
- 21.Wang S., Li X.M., Teuscher F., Li D.L., Diesel A., Ebel R., Proksch P., Wang B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006;69:1622–1625. doi: 10.1021/np060248n. [DOI] [PubMed] [Google Scholar]
- 22.Visagie C.M., Houbraken J., Frisvad J.C., Hong S.-B., Klaassen C.H.W., Perrone G., Seifert K.A., Varga J., Yaguchi T., Samson R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crystallographic Data of Compound 3 Have Been Deposited in the Cambridge Crystallographic Data Centre (CCDC 1529887) [(accessed on 11 May 2017)]; Available online: http://www.ccdc.cam.ac.uk/data_request/cif.
- 24.Sheldrick G.M. SADABS, Software for Empirical Absorption Correction. University of Göttingen; Göttingen, Germany: 1996. [Google Scholar]
- 25.Sheldrick G.M. SHELXTL, Structure Determination Software Programs. Bruker Analytical X-ray System Inc.; Madison, WI, USA: 1997. [Google Scholar]
- 26.Sheldrick G.M. SHELXL-97 and SHELXS-97, Program for X-ray Crystal Structure Solution and Refinement. University of Göttingen; Göttingen, Germany: 1997. [Google Scholar]
- 27.Al-Burtamani S.K.S., Fatope M.O., Marwah R.G., Onifade A.K., Al-Saidi S.H. Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol. 2005;96:107–112. doi: 10.1016/j.jep.2004.08.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.