Figure 1. 56% of GBMs develop resistance to CSF-1R inhibition in long-term preclinical trials.
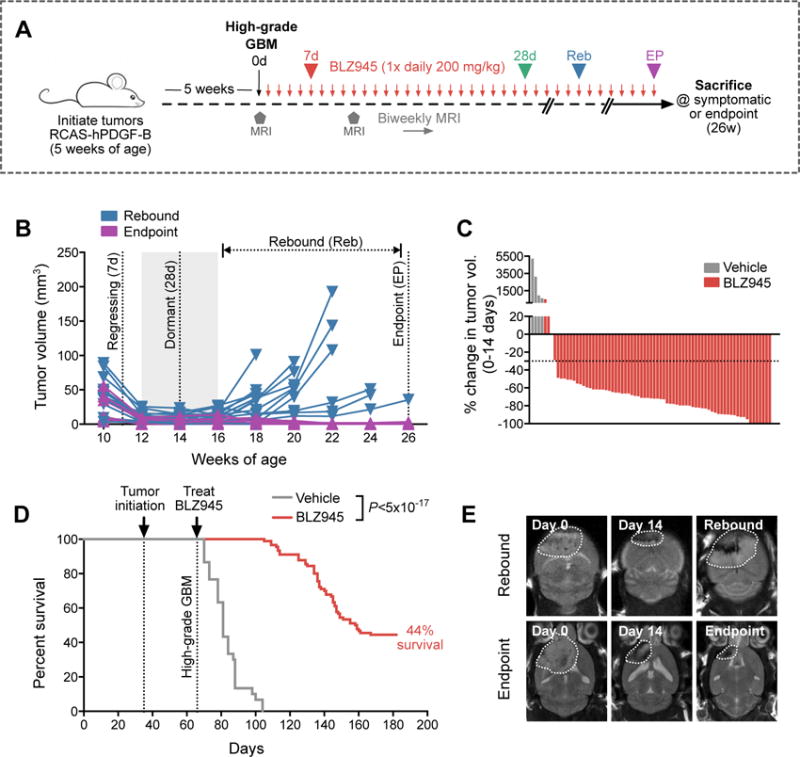
(A) Long-term trial design for testing BLZ945 efficacy in a PDGF-B-driven glioma (PDG) model. High-grade PDG tumors were treated with BLZ945 (200 mg/kg/d) or vehicle (20% captisol) and monitored by biweekly MRI for up to 26 weeks (defined endpoint; see methods) or until symptomatic. (B) Tumor volume curves from biweekly MRIs of long-term BLZ945 trials (n=90 animals treated, 23 representative curves are shown). Four key phases are indicated, including 7d (regressing tumor), 28d (dormant tumor), rebound (Reb; recurrent tumor, evaluated on a mouse-to-mouse basis by MRI), and endpoint (EP; stable regression at the 26-week endpoint). (C) Waterfall plots showing percent change in tumor volume between 0–14d in a representative subset of animals from Fig. 1D (BLZ945 n=71; vehicle n=4). (D) Kaplan-Meier of BLZ945-treated (n=90) versus vehicle-treated (n=30) mice bearing high-grade PDG tumors (Log-rank Mantel-Cox test, P<5×10−17). Median survival for vehicle-treated animals was 15d post-treatment initiation, while median survival for BLZ945-treated animals was 93d. (E) Representative MRI images over time of one mouse with a rebounded tumor (top row), and another mouse that had stable disease until EP (bottom row).
