Abstract
Introduction and Hypothesis
Pelvic floor muscle rehabilitation is a widely utilized, but often challenging therapy for pelvic floor disorders, which are prevalent in older women. Regimens involving the use of appendicular muscles, such as the obturator internus (OI), have been developed for strengthening of the levator ani muscle (LAM). However, changes that lead to potential dysfunction of these alternative targets in older women are not well known. We hypothesized that aging negatively impacts OI architecture, the main determinant of muscle function, and intramuscular extracellular matrix (ECM), paralleling age-related alterations in LAM.
Methods
OI and LAM were procured from three groups of female cadaveric donors (five per group): younger (20 – 40 years), middle-aged (41 – 60 years), and older (≥60 years). Architectural predictors of the excursional (fiber length, Lf), force-generating (physiological cross-sectional area, PCSA) and sarcomere length (Ls) capacity of the muscles, and ECM collagen content (measure of fibrosis) were determined using validated methods. The data were analyzed using one-way ANOVA and Tukey’s post-hoc test with a significance level of 0.05, and linear regression.
Results
The mean ages of the donors in the three groups were 31.2 ± 2.3 years, 47.6 ± 1.2 years, and 74.6 ± 4.2 years (P < 0.005). The groups did not differ with respect to parity or body mass index (P > 0.5). OI Lf and Ls were not affected by aging. Age >60 years was associated with a substantial decrease in OI PCSA and increased collagen content (P < 0.05). Reductions in OI and LAM force-generating capacities with age were highly correlated (r2 = 0.9).
Conclusions
Our findings of age-related decreases in predicted OI force production and fibrosis suggest that these alterations should be taken into consideration, when designing pelvic floor fitness programs for older women.
Keywords: Obturator internus, Muscle architecture, Aging, Pelvic floor rehabilitation
Introduction
Pelvic floor skeletal muscles (PFMs) are essential constituents of the female pelvic floor and their dysfunction is a critical component in the pathogenesis of pelvic floor disorders (PFDs), which include pelvic organ prolapse, and urinary and fecal incontinence. These morbid conditions affect almost a quarter of the US female population [1] and are accompanied by significant economic burden to the individuals and the health-care system [2–4]. Thus, substantial efforts have been made to develop effective rehabilitation strategies to improve PFM function.
Despite the clinical effectiveness of intensive PFM exercises, long-term adherence to PFM training is poor [5, 6]. Furthermore, many women are unable to properly contract the PFMs and require one-on-one supervised training, which includes internal manipulation of the PFMs through the vagina. The invasive approach and limited availability of therapists with expertise in pelvic floor rehabilitation dampen patients’ enthusiasm and reduce long-term compliance with PFM training programs. Importantly, a substantial proportion of women with compromised pelvic floor function are not able to properly contract the PFMs even after being instructed by an expert or receiving biofeedback [7, 8]. To circumvent the above challenges, regimens targeting the surrounding appendicular and trunk muscles, including the obturator internus (OI), have been developed to strengthen the PFMs. Recent studies indicate that exercise programs focused on the OI reduce symptomatic urinary incontinence and improve PFM strength in younger women [9, 10].
The prevalence of PFDs rises dramatically with age [1, 11], and thus older women comprise a large proportion of patients seeking treatment for these conditions. Age-related detrimental alterations in muscle architecture, the primary determinant of muscle function, and pathological accumulation of collagen or fibrosis in the intramuscular extracellular matrix (ECM), that is responsible for muscle stiffness and load-bearing capacity, have been shown to occur in the PFM [12]. These changes negatively impact muscle mechanical properties and are likely responsible for clinically observed levator ani muscle (LAM) weakness and impaired response to rehabilitation in older women [13–15], making alternative targets for PFM strengthening especially attractive in this population. However, aging also negatively impacts functionally important architectural parameters and the intramuscular ECM of the limb and trunk muscles [16]. Decrease in force-generating capacity and fibrosis with advancing age may account for the insensitivity of the appendicular muscles to rehabilitation in older individuals [17].
We hypothesized that, in analogy to other skeletal muscles, age-related changes in the architectural parameters, which deleteriously impact muscle predicted force production, and fibrosis take place in the OI muscle. Establishing aging effects on the OI will provide insight into the mechanics of muscle dysfunction in older women, which is important to consider when designing effective alternative PFM rehabilitation strategies in this population. The purpose of this study was to determine age-related alterations in the architectural design and collagen content of the OI muscle. The secondary aim was to determine if age-related changes in the OI and LAM are correlated. Probing muscle architecture is not feasible in living women; therefore we utilized human cadaveric specimens to enable our direct studies of OI intrinsic structural components.
Materials and methods
The study was exempt from institutional review board approval due to exclusion of living human subjects. Specimens were obtained through the University of Minnesota Bequest Body Donation Program. Donors with a history of pelvic irradiation, gynecological or colorectal malignancy, pelvic metastasis, connective tissue disorder, myopathy, surgery for PFDs, or colectomy were excluded. Epidemiological studies indicate that among patients seeking pelvic floor rehabilitation, a little over a third are less than 40 years old, with a similar proportion between 40 and 60 years of age, and more than 20 % are 65 years or older [18]. Therefore, we compared OI muscles among the three cadaveric groups (five per group): younger (20 – 40 years, middle-aged (41 – 60 years), and older (>60 years).
Architectural structure was maintained by perfusion fixing the OI and LAM in situ attached to the skeleton. Muscles were identified using origin-insertion pairs, with the obturator membrane and the medial surface of the greater trochanter as the origin and insertion of the OI, respectively, and were harvested and weighed. The OI were divided into cephalad and caudate regions, while the individual components of the LAM complex were divided into three regions, as previously described [12]. The following architectural parameters were determined using previously validated methods [12]: muscle mass, fiber length (Lf), a prognostic indicator of muscle excursion and contractile velocity, sarcomere length (Ls), which determines force produced upon stimulation, and physiological cross-sectional area (PCSA), a predictor of isometric force-generation capacity.
Briefly, three fiber bundles, consisting of about 10 – 20 fibers, were dissected from each region for measurement of Lf. Myofibers were microdissected under ×60 magnification (Leica MZ16; Meyer Instruments Inc., Houston, TX) for determination of Ls by laser diffraction. The number of sarcomeres in series within fibers, derived from Ls and Lf, was used to normalize fiber length (Lfn) to account for potential differences in length of the specimens at the time of fixation. PCSA was calculated using an optimal human sarcomere length of 2.7 μm. Skeletal muscle collagen content, which is the main component of intramuscular ECM, was determined from the hydroxyproline concentration, quantified using a validated protocol [19].
Statistical analyses were performed using GraphPad Prism version 6.00 (GraphPad Software, San Diego, CA). One-way analysis of variance, followed by pair-wise comparisons with Tukey’s range test and the unpaired Student’s t test were used to determine the effects of aging and to compare variables among groups. Correlations between significantly different age-related architectural parameters of the OI and LAM in the same donors were determined by linear regression. The significance level (α) was set to 5 %. To obtain 80 % power (1 − β) to detect a 20 % difference, the sample size of five per group was calculated utilizing the equation: n = 16 × (CV)2[(ln(1 − δ)]2 with an experimentally determined variability of 11.5 % where n is the sample size, δ is the difference desired, and CV is the coefficient of variation [20]. All data were screened for normality and skew in order to satisfy the assumptions of the parametric tests used. The results are presented as means ± standard error of the mean (SEM), except where noted.
Results
The mean ages of the younger (31.2 ± 2.3 years), middle-aged (47.6 ± 1.2 years), and older (74.6 ± 4.2 years) donor groups differed significantly (P < 0.005), permitting a meaningful quantification of an the impact of age (Table 1). Parity and body mass index were similar among the groups (P > 0.5; Table 1), eliminating potential confounding effects. Inspection of gross anatomy did not reveal detachments of the PFMs from the obturator fascia at the arcus tendineus levator ani in any of the 15 specimens.
Table 1.
Demographic variables of cadaveric donors (five donors per group)
| Age (years), mean (SEM) | BMI (kg/m2), mean (SEM) | Parity, median (range) | ||
|---|---|---|---|---|
| Group | Younger | 31.2 (2.3) | 22.9 (2.3) | 0 (0 – 2) |
| Middle-aged | 47.6 (1.2) | 20.2 (1.7) | 1 (0 – 2) | |
| Older | 74.6 (4.2) | 20.3 (1.9) | 1 (0 – 5) | |
| P valuea | Younger vs. middle-aged | 0.005 | 0.62 | 0.89 |
| Younger vs. older | <0.0001 | 0.64 | 0.53 | |
| Middle-aged vs. older | <0.0001 | 0.99 | 0.79 |
P values were derived from Tukey’s pair-wise comparisons, following one-way analysis of variance, with the significance level set to 5 %.
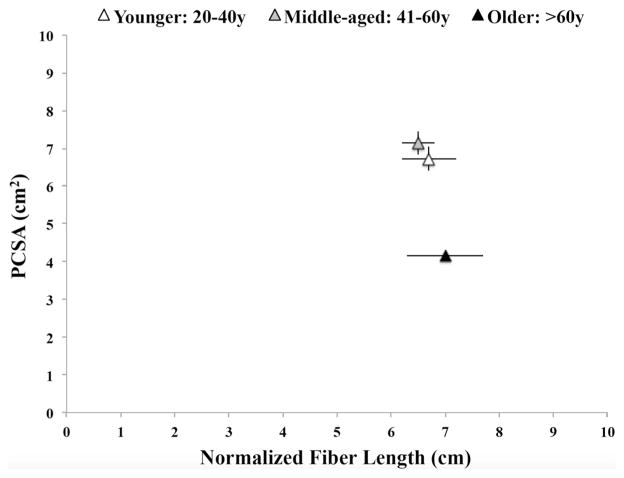
OI muscle mass was almost identical in the younger (43.7 ± 3.5 g) and middle-aged (44.8 ± 4.9 g) groups (P = 0.9). Muscle mass in the older group, although lower, did not differ significantly from that in the younger group (29.5 ± 3.6 g vs. 43.7 ± 3.5 g, P = 0.07) or middle-aged group (29.5 ± 3.6 g vs. 44.8 ± 4.9 g, P = 0.05). The relationship between OI force-producing and excursion capacity, as determined by its PCSA and Lfn [21], is shown for each group in Fig. 1. OI PCSA remained unchanged in the middle-aged group (7.1 ± 0.9 cm2) compared with the younger group (6.7 ± 0.3 cm2, P = 0.9). OI PCSA in the older group (4.1 ± 0.3 cm2), on the other hand, was substantially lower than in the younger (P = 0.02) and middle-aged groups (P = 0.01; Fig. 1). Lfn did not differ between the groups: 6.7 ± 0.5 cm in the younger group, 6.5 ± 0.3 cm in the middle-aged group, and 7.0 ± 0.7 cm in the older group (P > 0.5). OI Ls, which is within 10 % of the in vivo length in fixed muscles, was similar in all groups: 2.3 ± 0.1 μm in the younger group, 2.4 ± 0.2 μm in the middle-aged group, and 2.5 ± 0.1 μm in the older group (P > 0.2).
Fig. 1.
Normalized fiber lengths in relation to physiological cross-sectional areas (PCSA) of obturator internus muscles in specimens from the age groups
OI collagen content did not differ between the younger and middle-aged groups: 10.0 ± 1.2 μg/mg and 11.3 ± 1.4 μg/mg, respectively (P = 0.8). OI intramuscular collagen content was 15.6 ± 1.8 μg/mg in the older group, which was significantly higher than in the younger group (P = 0.04), but not the middle-aged group (P = 0.16).
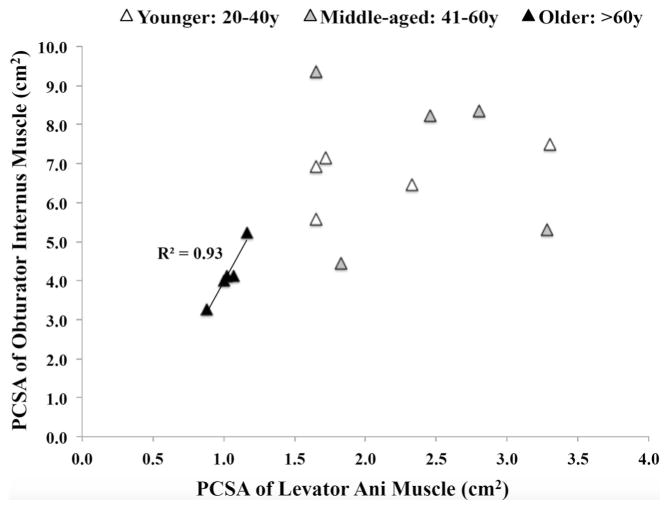
Even though childbirth is not known to detrimentally affect the OI muscle, we also compared PCSA between specimens from eight vaginally nulliparous and seven vaginally parous donors. PCSA was not significantly different between the nulliparous and parous donors: 6.5 ± 0.7 cm2 and 5.4 ± 0.6 cm2, respectively (P = 0.2). Age-related decrease in OI PCSA paralleled changes in the LAM, including the iliococcygeus and pubovisceralis. Similar to the OI, the LAM PCSA did not differ between the younger and the middle-aged groups (2.1 ± 0.3 cm2 and 2.4 ± 0.3 cm2, respectively, P = 0.7), but was significantly lower in the older group (1.0 ± 0.05 cm2) than in the younger group (P = 0.02) and the middle-aged group (P = 0.006). The PCSA of the OI and the LAM were significanly correlated in the older group (r2 = 0.9; Fig. 2), but not in either the younger group (r2 = 0.3) or the middle-aged group (r2 = 0.04).
Fig. 2.
Physiological cross-sectional area (PCSA) of the levator ani muscle in relation to that of the obturator internus muscle. The R2 value of 0.93 was derived from linear regression
Discussion
The attachment of the LAM to the obturator fascia along the lateral wall of the pelvis forms the arcus tendineus levator ani. It is, therefore, feasible that mechanical loading of the levator ani can be achieved by augmenting the tension in the fascial connections between these muscles during OI contraction. This likely accounts for the improvements in PFM function through OI-targeted exercise programs in younger women [9]. Unfortunately, we do not yet know if this alternative method for PFM rehabilitation is suitable for older women with PFDs. Clinical studies employing magnetic resonance imaging demonstrate that the OI muscles atrophy with age [22]. However, radiological investigations assess cross-sectional area, which is a poor proxy measure for muscle force-generating capacity [23]. Furthermore, volumetric measures of smaller appendicular muscles with the line of action oriented parallel to the transverse imaging plane, such as the OI, are susceptible to partial volume effects and segmentation errors [24]. To circumvent these limitations, we sought to directly identify structural changes that potentially govern functional alterations of the OI muscle as a consequence of aging by comparing architectural parameters of human cadaveric OI specimens among three age groups.
Our results demonstrate that OI architectural design was unaltered in the middle-aged group relative to the younger group. On the other hand, a dramatic decrease in OI force-generating capability and muscle fibrosis was observed in the older group, supporting our hypothesis. The extent of the age-related decrease in OI PCSA exceeded the decrease in muscle mass. These findings are consistent with clinical reports of muscle weakness in women >60 years of age, which surpasses the age-related decrease in muscle mass and in large part is due to aging changes in muscle architecture [25, 26].
The function of the OI is often described as a lateral rotator of the extended hip and an abductor of the flexed hip. However, the OI shortens as the hip moves from flexion to extension, indicating its important role in providing and maintaining stability of the hip during weight bearing and propulsive motion [27]. Thus, more functional load-bearing exercises combining hip extension, external rotation and abduction appear to be better suited for OI strengthening than the commonly utilized non-weight-bearing training with a flexed hip [9]. PFM rehabilitation regimens that maximize the strength of OI contraction are likely to be especially important, when the predicted force-generating capacity of the OI is reduced with age.
The limitations of this study are related to the use of fixed cadaveric specimens, the variability in body position at the time of fixation, and the possible impact of additional factors, such as age-related neuropathy, on muscle function. However, previously it has been demonstrated that the architectural parameters are not altered by the differential topography of fixed, compared with in vivo muscles [28]. To account for potential differences in fiber length due to non-uniform hip position at the time of fixation, fiber length was normalized to sarcomere length in each specimen, allowing meaningful comparisons [29]. We are also confident that the main impact of aging on muscle function can be deduced from changes in muscle architectural parameters. Support for this comes from studies demonstrating the myogenic origin of age-related alterations in other limb muscles [30]. Furthermore, it is well established that the decrease in muscle strength with age is due to marked age-related alterations in muscle architecture resulting from the loss of sarcomeres in parallel and in series [16, 26, 31].
Conclusion
Our results reveal that age greater than 60 years is associated with fibrosis and a substantial decrease in predicted force production of the OI muscle, in correlation with reduced force-generating capacity of the LAM. These changes would most likely diminish the observed efficacy of the external hip rotator exercise regimens in older women and should be taken into consideration when prescribing a conservative exercise-based treatment plan for older patients with PFDs. In light of the current findings, it may be necessary to identify alternative muscle groups to augment PFM training in this population.
Acknowledgments
The authors thank the individuals who donated their bodies to the University of Minnesota’s Anatomy Bequest Program for the advancement of education and research.
Funding The authors gratefully acknowledge funding by NIH grants 1R03HDO75994 and K12HD001259 for the conduct of this research.
Footnotes
Compliance with ethical standards
Conflicts of interest None.
References
- 1.Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123:141–148. doi: 10.1097/AOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLancey JO. Pelvic organ prolapse: clinical management and scientific foundations. Clin Obstet Gynecol. 1993;36:895–896. [Google Scholar]
- 3.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–651. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 4.Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 5.Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstet Gynecol. 2005;105:999–1005. doi: 10.1097/01.AOG.0000157207.95680.6d. [DOI] [PubMed] [Google Scholar]
- 6.Glazener CM, Herbison GP, MacArthur C, Grant A, Wilson PD. Randomised controlled trial of conservative management of postnatal urinary and faecal incontinence: six year follow up. BMJ. 2005;330:337. doi: 10.1136/bmj.38320.613461.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bump RC, Hurt WG, Fantl JA, Wyman JF. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165:322–327. doi: 10.1016/0002-9378(91)90085-6. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 8.Henderson JW, Wang S, Egger MJ, Masters M, Nygaard I. Can women correctly contract their pelvic floor muscles without formal instruction? Female Pelvic Med Reconstr Surg. 2013;19:8–12. doi: 10.1097/SPV.0b013e31827ab9d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuttle LJ, DeLozier ER, Harter KA, Johnson SA, Plotts CN, Swartz JL. The role of the obturator internus muscle in pelvic floor function. J Womens Health Phys Therap. 2016;40:15–19. [Google Scholar]
- 10.Jordre BS, Schweinle W. Comparing resisted hip rotation with pelvic floor muscle training in women with stress urinary incontinence: a pilot study. J Womens Health Phys Therap. 2014;38:81–89. [Google Scholar]
- 11.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alperin M, Cook M, Tuttle LJ, Esparza MC, Lieber RL. Impact of vaginal parity and aging on the architectural design of pelvic ploor muscles. Am J Obstet Gynecol. 2016;215:312.e1, 312.e9. doi: 10.1016/j.ajog.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherburn M, Bird M, Carey M, Bø K, Galea MP. Incontinence improves in older women after intensive pelvic floor muscle training: an assessor-blinded randomized controlled trial. Neurourol Urodyn. 2011;30:317–324. doi: 10.1002/nau.20968. [DOI] [PubMed] [Google Scholar]
- 14.Lewicky-Gaupp C, Brincat C, Yousuf A, Patel DA, Delancey JO, Fenner DE. Fecal incontinence in older women: are levator ani defects a factor? Am J Obstet Gynecol. 2010;202:491.e1–491.e6. doi: 10.1016/j.ajog.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Pelvic floor muscle function in a general female population in relation with age and parity and the relation between voluntary and involuntary contractions of the pelvic floor musculature. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1497–1504. doi: 10.1007/s00192-009-0978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol (1985) 2003;95:2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- 17.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Wang YC, Hart DL, Mioduski JE. Characteristics of patients seeking outpatient rehabilitation for pelvic-floor dysfunction. Phys Ther. 2012;92:1160–1174. doi: 10.2522/ptj.20110264. [DOI] [PubMed] [Google Scholar]
- 19.Alperin M, Lawley DM, Esparza MC, Lieber RL. Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. Am J Obstet Gynecol. 2015;213:191.e1–191.e7. doi: 10.1016/j.ajog.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuttle LJ, Ward SR, Lieber RL. Sample size considerations in human muscle architecture studies. Muscle Nerve. 2012;45:743–745. doi: 10.1002/mus.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Morris VC, Murray MP, Delancey JO, Ashton-Miller JA. A comparison of the effect of age on levator ani and obturator internus muscle cross-sectional areas and volumes in nulliparous women. Neurourol Urodyn. 2012;31:481–486. doi: 10.1002/nau.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Day MK, Lee PL, et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992;10:928–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- 24.Handsfield GG, Meyer CH, Hart JM, Abel MF, Blemker SS. Relationships of 35 lower limb muscles to height and body mass quantified using MRI. J Biomech. 2014;47:631–638. doi: 10.1016/j.jbiomech.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist–antagonist coactivation account for differences in torque between young and older women. Muscle Nerve. 2002;25:858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- 26.Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol. 2004;92:219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 27.Vaarbakken K, Steen H, Samuelsen G, Dahl HA, Leergaard TB, Nordsletten L, et al. Lengths of the external hip rotators in mobilized cadavers indicate the quadriceps coxa as a primary abductor and extensor of the flexed hip. Clin Biomech (Bristol, Avon) 2014;29:794–802. doi: 10.1016/j.clinbiomech.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Janda TN, van der Helm FCT, de Blok SB. Measuring morphological parameters of the pelvic floor for finite element modelling purposes. J Biomech. 2003;36:749–757. doi: 10.1016/s0021-9290(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 29.Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J Exp Biol. 2005;208:3275–3279. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- 30.Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol (1985) 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- 31.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol (1985) 2001;91:1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]