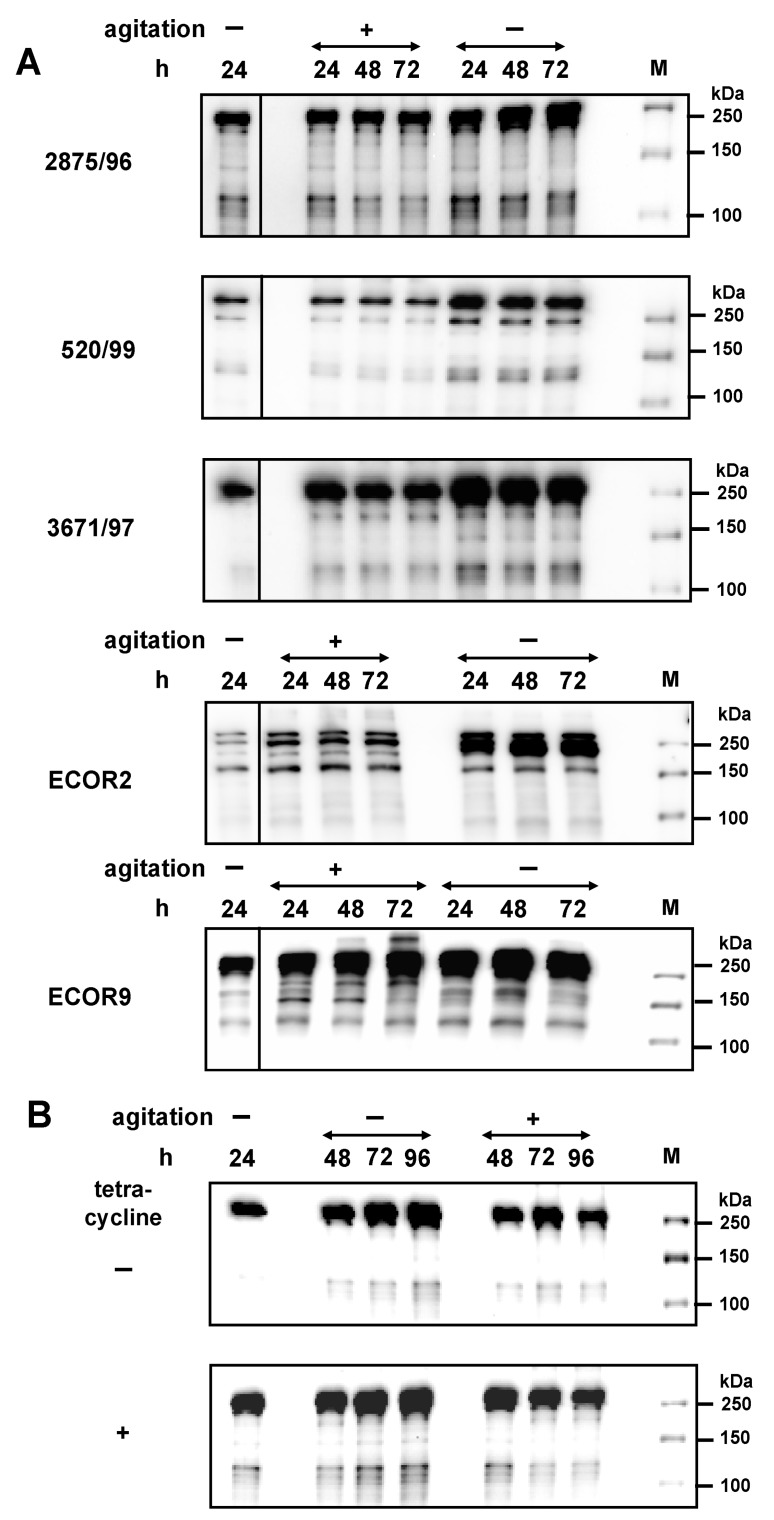
Figure 5.
Once expressed, EibG proteins demonstrate high stability during prolonged incubation. For the adequate synthesis of EibG, strains 2875/96, 0520/99, and 3671/97 were incubated statically in LB broth without agitation (−) for 24 h. (A) These cultures were re-inoculated into a fresh medium at a 1:100 dilution followed by incubation with (+) and without (−) shaking. Aliquots were harvested after indicated time periods. Proteins (4 µg per lane) were immunoblotted and EibG were detected using human IgG Fc fragment antibody. (B) After static growth for 24 h and expression of EibG, the culture of STEC strain 2875/96 was aliquoted and incubation was extended with (+) and without (−) shaking. To inhibit protein and EibG synthesis, one aliquot was laced with tetracycline (20 µg/mL) (+). Cells were harvested at the indicated periods of time and proteins from the aliquots were immunoblotted. Molecular masses of standard proteins are indicated.