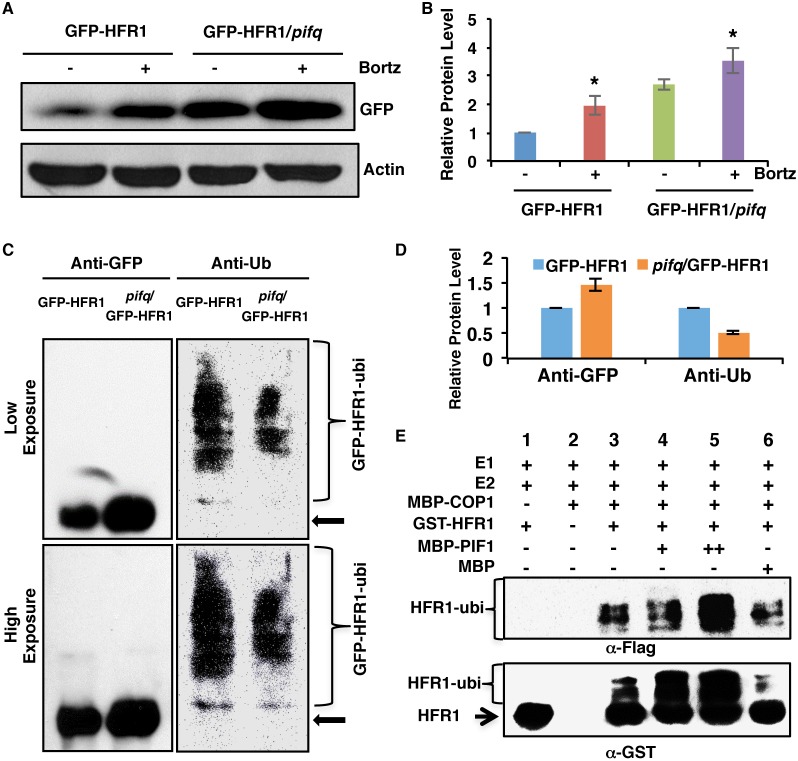
Fig. 5.
PIF1 promotes HFR1 degradation in a ubiquitylation-dependent manner. (A) Immunoblot shows the GFP-HFR1 protein level in GFP-HFR1 and GFP-HFR1/pifq backgrounds. Total protein was extracted from 4-day-old seedlings grown in darkness. One batch of seedlings was pretreated with 40 mM Bortezomib (Bortz) for 3 h before protein extraction. The blot was probed with anti-GFP or anti-actin antibodies. (B) Quantification of GFP-HFR1 protein level using actin as a control. Asterisks indicate statistically significant differences compared with non-Bortezomib treatment for GFP-HFR1 and GFP-HFR1/pifq, respectively (P<0.05). Error bars indicate s.d. (n=4). (C) The protein level of GFP-HFR1 is higher but the ubiquitylation level of GFP-HFR1 is lower in the pifq compared with the GFP-HFR1 background in darkness in vivo. Sample preparation is as described in A. GFP-HFR1 was immunoprecipitated using anti-GFP antibody, and then separated on 8% SDS-PAGE gels and probed with anti-GFP (left) or anti-Ub (right) antibodies. The top and bottom panels are low and high exposures, respectively. The arrow indicates the GFP-HFR1 size. (D) Quantification of GFP-HFR1 and GFP-HFR1-ubi levels for the blot shown in C by ImageJ. The GFP-HFR1 and GFP-HFR1-ubi levels were set as 1, respectively. Error bars indicate s.d. (n=3). (E) PIF1 promotes the ubiquitylation of HFR1 by COP1 in vitro. In vitro ubiquitylation assay was performed using MBP-COP1 as E3 ubiquitin ligase, GST-HFR1 as a substrate, Flag-ubiquitin, UBE1 (E1), UbcH5b (E2) and increasing concentrations of MBP-PIF1. MBP was used as a control. Ubiquitylated GST-HFR1 was detected by anti-Flag antibody (top panel) and anti-GST antibody (bottom panel). The arrow indicates non-ubiquitylated GST-HFR1.