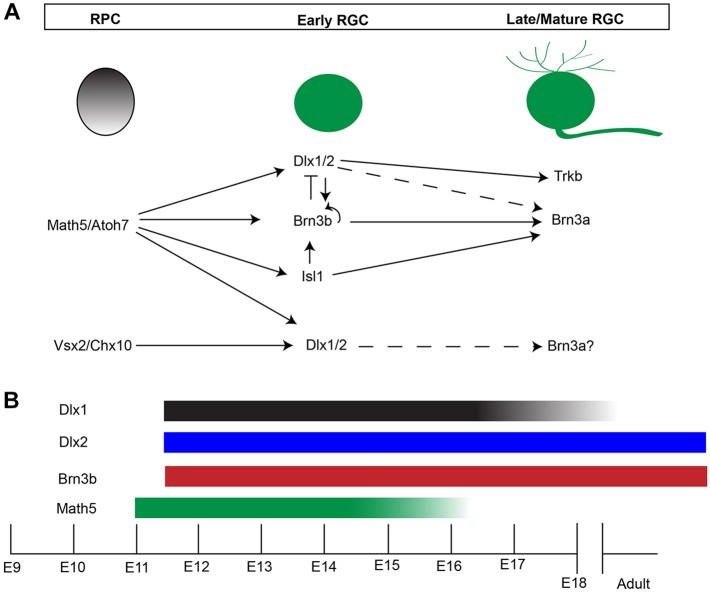
Fig. 10.
A model for transcriptional regulation of RGC differentiation. (A) Loss of DLX2-expressing cells in E13 Atoh7 KO supports Dlx1/Dlx2 participation in a regulatory pathway downstream of Atoh7, where, along with Brn3b and Isl1, Dlx1/Dlx2 then co-regulates the genes required for RGC differentiation and survival. In addition, cross-regulation of these factors is observed. Loss of Dlx2, Brn3b or Isl1 individually does not affect initial RGC genesis, suggesting that a combination/co-regulation of these factors and/or other unidentified factors are responsible for RGC fate specification. In later development, loss of Dlx2, Brn3b and Isl1 all result in increased RGC death, supporting the requirement of these factors for RGC survival by controlling the expression of genes necessary for this process, including transcriptional control of Brn3a by DLX2 (Q.Z. and D.D.E., unpublished), ISL1 and BRN3B (Pan et al., 2008), and DLX2-mediated regulation of Trkb (de Melo et al., 2008). Solid lines, established regulation; broken lines, proposed regulation. (B) Atoh7 expression begins at ∼E11, preceding Dlx1/Dlx2 and Brn3b. Atoh7 and Dlx1 expression are both downregulated after E16.5, with neither detected after P0. Dlx2 and Brn3b expression starts at E11.5 and persists to adulthood.