Abstract
Purpose:
Evidence regarding the association of smoking with various forms of chronic musculoskeletal pain is vast, but that with temporomandibular disorders (TMD) is scarce.
Aims:
The aims of this study are to evaluate the effect of smoking status (SS) and nicotine dependence (ND) on TMD pain intensity and treatment outcome in an Indian population with TMD.
Subjects and Methods:
Nine hundred and sixty-two patients with TMD were selected for this longitudinal cohort study. Lifetime SS was evaluated and patients were classified as current smokers (YS), former smokers (FS), or nonsmokers (NS). The Fagerstrom test was used to evaluate the ND of YS. Pain intensity was evaluated using visual analog scale scores. Six months posttreatment, the pain intensity was again recorded. The effect of treatment was evaluated using a global transition outcome measure and categorized as treatment success or failure. A minimum 30% reduction in pain was used as a criterion for categorizing patients as those who had gotten “better.” Data obtained from the study were compared using Chi-square tests, paired samples t-tests, and one-way ANOVA tests. The criterion for statistical significance for all analyses was set at P = 0.05.
Results:
Among groups of SS, YS showed the maximum pain intensity at baseline and posttreatment. The outcome of treatment was most successful in NS and least in FS. The number of patients who had gotten “better” after treatment was significantly highest in NS. There was no significant difference between groups of ND with respect to pain intensity, treatment outcome, or “better” patients.
Conclusions:
Among Indian patients with TMD, smokers reported significantly greater pain intensity and poorer response to treatment than NS. Pain intensity or treatment outcome was independent of ND.
Keywords: Pain assessment, temporomandibular joint disorders, tobacco smoking, tobacco use disorder
INTRODUCTION
Smoking tobacco has been associated with several chronic pain conditions. The use of tobacco has been established as a risk factor for cardiovascular disease, chronic obstructive pulmonary disease, spectrum of malignancies,[1,2] and more recently, periodontal disease[3,4] and osteoporosis.[5,6] An association between smoking and distribution of chronic pain in multiple musculoskeletal sites has been found but only with the involvement of the neck or the lower back.[7,8] Interestingly, very few studies have described smoking as a risk or exacerbating factor in locations other than the low back region. It was Jay who first suggested the exploration of the effect of smoking on the genesis of symptoms of chronic fatigue syndrome, fibromyalgia, and temporomandibular disorders (TMDs).[9]
Since then, a few studies have examined the effect of smoking on TMD. Two retrospective chart reviews have revealed that smokers with TMD reported higher pain intensity and life interference from pain than nonsmokers (NS) with TMD.[10,11] On the other hand, a prospective cohort study spanning 6 years evaluated whether smoking influenced the presence and/or development of signs and symptoms of TMD. No differences were found between the cohort of smokers and the cohort of NS with regard to the presence or development of TMD signs and symptoms.[12] Another relatively recent study has concluded that women younger than 30 years with temporomandibular disorders (TMDs) were four times more likely to be current smokers (YS) or former smokers (FS) than women of any age who had no clinical signs of TMD.[13]
There have been strong indications that smokers with chronic pain tend to report higher pain severity, greater functional disability, more anxiety, depression, and sleep disturbances.[14,15,16,17,18,19] One study has concluded that smokers with TMD not only reported higher pain severity than NS with TMD but also were at higher risk for factors that may adversely affect treatment outcomes.[20] In contrast to this, in another study on a series of patients undergoing multidisciplinary treatment for chronic pain, immediate treatment effects for a variety of outcome measures were similar or significantly better in smokers compared with NS.[21] Hence, the role of smoking on TMD pain intensity and treatment outcome appears to be a controversial one.
Smoking may influence pain either through allergic or inflammatory pathways or both.[13] It is well known that smoking is associated with the production of pro-inflammatory cytokines and inhibition of anti-inflammatory cytokines. Smoking increases sensitization to allergens[22] and facilitates permeability of the respiratory epithelium.[23] Serum immunoglobulin E levels are higher in smokers than NS, and these levels decrease dramatically with age.[24] The potential relationship between smoking and chronic pain intensity has been explored in several other studies, and the various possible biochemical mechanisms have been explained.[25,26,27] The way that smoking can affect muscular and joint structures to cause an increase of TMD pain intensity is probably related to its effect on muscle metabolism,[28,29,30] disk degeneration,[31,32] and other biological phenomena.[33,34] Smoking has also been described as a coping strategy for chronic pain.[35,36]
A substantial proportion of the Indian population has been documented with a current or past smoking habit.[37] In a study to provide nationally representative aggregate prevalence estimates of tobacco consumption by different socioeconomic and demographic characteristics in India, it was found that 16% of the population 15 years or older (29.3% men and 2.3% women) smoked tobacco. This translates to almost 102 million people – 94 million men and 8 million women in India.[38]
It is a well-established fact that TMD is quite common, affecting up to 28% of the population worldwide.[39] Studies across the globe have also shown that females have TMD signs and symptoms more frequently than males,[40,41,42] the most common problem in both genders being pain.[43] Data regarding TMD prevalence in Indian populations have been scarce. The prevalence of signs and symptoms of TMD in an Indian population according to Gopal et al. was found to be 52%.[44] Modi et al. found TMD prevalence as high as 68.6% in an Indian student population, with a higher number of females being affected than males.[45]
To date, there are no published data regarding an association, if any, between smoking, chronic TMD pain intensity, and treatment outcome in an Indian population of TMD patients. Accounting for smoking is important in TMD research not only because of smoking's potential causal role but also because smoking may account for effects of other TMD risk factors including perceived stress, anxiety, and depression.[46,47,48,49,50] Furthermore, an association has been found between cigarette smoking and bruxism[51,52] which, in turn, is frequently associated with TMD.[52,53] Therefore, smoking may be a key prognostic factor in the treatment of chronic TMD pain.
In view of the above, the objectives of the present study were to evaluate the effect of smoking status (SS) and nicotine dependence (ND) on TMD pain intensity and treatment outcome in an Indian population with TMD.
SUBJECTS AND METHODS
The study was approved by the Institutional Human Ethics Committee (Ethical Clearance Number: GMERS/MCG/EC/46).
Sample size determination
Between October 2012 and December 2014, a total of 18,362 consecutive patients, who reported to the Dentistry Department of GMERS Medical College, Civil Hospital, Gandhinagar, were screened at their first visit. A sample size of 784 patients was calculated to be sufficient to detect a margin of error no more than 3.5% between groups, with 95% confidence level and a 5% significant level. Medical and dental histories were recorded for all patients before a clinical examination. A total of 1263 patients were selected as eligible patients, of which 1124 gave their consent to be a part of the study. Out of these, 162 patients opted out of the study at some point or the other, leaving us with a study population of 962 patients.
Inclusion and exclusion criteria
Eligible candidates were those with painful TMD. TMD diagnosis was recorded according to the Axis I of the Research Diagnostic Criteria (RDC) for TMD by Dworkin and LeResche[54] and patients were classified as having myofascial pain (RDC/TMD Group I) if their primary and secondary (if present) diagnoses were of myogenic origin. They were classified as having arthralgia/osteoarthritis (RDC/TMD Group III) if their primary and secondary (if present) diagnoses were of painful arthrogenous origin. Patients with osteoarthrosis (by definition, pain-free) as a primary diagnosis were excluded from the study, whereas patients with osteoarthrosis as a secondary diagnosis were included if their primary diagnosis was arthralgia/osteoarthritis (note that this could be the contralateral joint). In this group, a secondary diagnosis of disc displacement (RDC/TMD Group II) was also allowed. Finally, patients with a primary and secondary pain-related diagnosis, one from each RDC/TMD Group I and RDC/TMD Group III, comprised the mixed TMD group. In summary, cases were people who reported a 6-month history of pain in the temporomandibular structures, with at least 5 days of such pain in the month preceding the examination and where examiners found at least three muscle groups in the temporomandibular region that were tender to palpation or jaw maneuver. The threshold of 6 months was chosen to be consistent with the classification of chronic pain for research purposes described in the IASP Task Force on Taxonomy.[55] Other criteria for inclusion in the study population were that the patients had to be between 18 and 60 years of age, had to be of Indian origin, had to want treatment for their TMD condition, and had to give an informed consent on the day of the initial visit and to allow the use of their data for research purpose.
Patients with diagnoses of any one of diabetes, kidney disease, heart failure, chronic respiratory disease, epilepsy or seizure disorder, or high blood pressure not controlled with medication, or psychological disorders were excluded. Also excluded were those who were pregnant, nursing, undergoing orthodontic treatment, renal dialysis, radiation or chemotherapy as well as persons with trauma or surgery on the head, face, or neck within the last 6 months. Patients on presentation for the study who were currently on any type of prescribed medication or receiving treatment for their headache complaint, patients presenting with acknowledged or identified diagnosis of TMD were also not eligible for the study.
Data collection
The study included a questionnaire to be filled by the patients. Age and gender of the patients were recorded and they were then divided into three age categories: up to 30 years, from 31 to 50 years, and more than 50 years.
Pretreatment (baseline) pain assessment
The intensity of pain due to TMD was derived from a 100-mm visual analog scale (VAS) asking patients to report average pain over the past month. This was considered as the baseline pain intensity score.
Grouping of study population
Lifetime SS was evaluated with the question widely used in major population surveys including the National Health Interview Survey, the National Health and Nutrition Examination Survey, and the Behavioral Risk Factor Surveillance Survey: Have you smoked at least 100 cigarettes in your entire life?[13] Those with a negative response were classified as lifetime NS. Those with an affirmative response were further classified as YS or FS).
The questionnaire also contained the Fagerstrom Test for Nicotine Dependence (FTND), a self-reported measure of ND for YS.[56,57,58,59,60] The 6-item FTND is a revised and abbreviated version of the Fagerstrom Tolerance Questionnaire.[56,57,58] The questionnaire assessed the clinical indicators of dependence, such as the number of cigarettes smoked per day, and the level of difficulty in refraining from smoking when it is not allowed. Scores from the FTND range from 0 to 10, with 0–2 indicating very low dependence (VLD), 3–4 indicating low dependence (LD), 5 indicating moderate dependence (MD), 6–7 indicating high dependence (HD), and 8–10 indicating very HD. For the purpose of simplicity, scores 5–7 were clubbed into one category in this study, to indicate MD to HD.
Treatment protocol
Primary treatment was provided based on each individual's needs, and appropriate treatment approaches were rendered to effect improvement. Treatment was rendered to all patients either by therapeutic exercises of the jaw alone or in combination with stabilization splint therapy, as per the standard recommendations[61,62,63] and evidence-based clinical practice guidelines[49] for the management of the specific TMD conditions. Most patients also received some type of pharmacological intervention.
Patients were instructed to perform a standardized program for masticatory muscle exercises as described by Carlsson and Magnusson.[50,64] Stabilization splints were made using heat cure acrylic resin. The splints ensured occlusal contact of all mandibular teeth in centric relation, anterior guidance for disocclusion in protrusion, and canine guidance for disocclusion in lateral movements. The patients were instructed to wear the appliances while sleeping at night for a minimum of 12 h.[50,65] The appliances were adjusted at regular follow-up intervals, and after 10 weeks, the patients were advised to gradually reduce wear of the appliances up to a minimum of 8 h a day.
Treatment outcome evaluation
At the end of 6 months posttreatment, the effect of treatment using a global transition outcome measure was evaluated. Each patient was verbally asked the question, “Has your pain gotten better, stayed the same, or gotten worse since you began treatment?” The responses were further subdivided with a better response suggesting “successful treatment” and responses of the same or worse grouped into “treatment failure.”
Posttreatment pain assessment
The intensity of pain was once again derived from a 100-mm VAS asking patients to report average pain over the past month. This was considered as the posttreatment pain intensity score.
In addition, to evaluate changes in pain intensity, a minimum 30% reduction in pain, as measured using the baseline and posttreatment VAS scores, was used as a criterion for improvement.[66,67] Patients who met this criterion were categorized as those who had gotten “better.”
For all individuals taking part in the study, a single specialist in stomatognathic physiology performed the screening for TMD, clinical examination, treatment planning, recording of VAS scores, and evaluation of global transition outcome. The same specialist delivered and adjusted the appliances at regular follow-up intervals and also gave the instructions for masticatory muscle exercises, as per the treatment plan. Any information pertaining to the SS and ND that was collected from the patients’ questionnaires was not revealed to that specialist at any point of time during the conduct of the study.
Statistical analyses
All statistical analyses were completed using the SPSS statistical program. First, a set of Chi-square tests examined age and sex differences within different groups of SS as well as ND. Paired samples t-tests were done to compare baseline and posttreatment pain intensity VAS scores of all the patients. One-way ANOVA tests were used to compare the mean TMD pain intensity VAS values between different groups of SS and ND, at baseline as well as posttreatment. Post hoc multiple comparisons among groups were performed with Tukey Honestly Significant Difference (HSD) tests. Similarly, the mean reduction in pain intensity VAS scores between different groups of SS and ND was compared.
Finally, Chi-square tests were used to compare treatment outcome and the number of patients showing improvement (according to the 30% cutoff criterion) within different groups of SS and ND. The criterion for statistical significance for all analyses was set at P = 0.05.
RESULTS
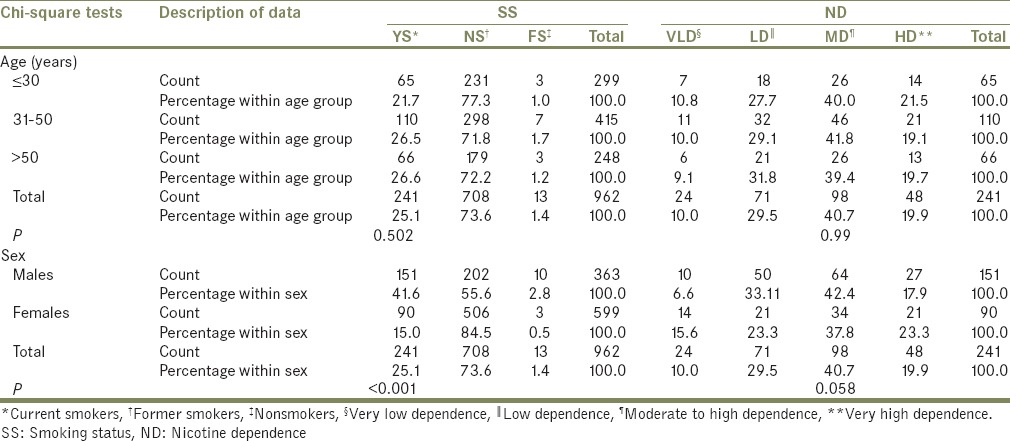
A total of 962 TMD patients participated in the study, of which majority of the patients were aged 31–50 years (n = 415), were female (n = 599), and were NS (n = 708). YS (n = 241) comprised 25.1% of the population, while FS (n = 13), the remaining 1.4% [Table 1]. Most of the patients in YS showed moderate to high ND (MD; n = 98), followed by low ND (LD; 71). Most of the YS and FS were male (n = 151 and n = 10, respectively), while most of the NS were female (n = 506) and only this relationship between sex and SS was statistically significant (P = 0.047).
Table 1.
Age and sex distribution of study population among different groups of smoking status and nicotine dependence
The mean difference of 18.728 between baseline and posttreatment VAS scores for TMD pain intensity was statistically significant [P ≤ 0.001, Table 2].
Table 2.
Comparison between baseline and posttreatment temporomandibular disorder pain intensity visual analog scale scores

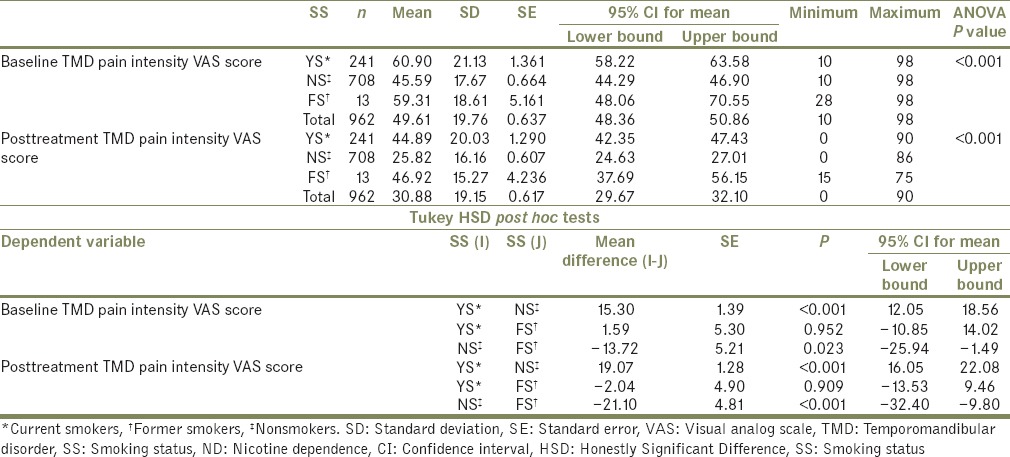
The mean TMD pain intensity VAS scores between the three groups of SS were compared and the difference was found to be statistically significant at baseline, (F [2959] = 62.65, P ≤ 0.001) as well as posttreatment [F (2959]=116.24, P ≤ 0.001, Table 3]. At baseline, post hoc comparisons using the Tukey HSD test indicated that the mean VAS score for YS (mean = 60.90, standard deviation [SD] =21.13) was significantly higher than that of NS (mean = 45.59, SD = 17.67). There was also a significant difference between mean VAS scores of NS and FS (mean = 59.31, SD = 18.61). Posttreatment too, post hoc tests showed that the mean VAS score for the YS (mean = 44.89, SD = 20.03) was significantly higher than that of NS (mean = 25.82, SD = 16.16), and mean VAS score for FS (mean = 46.92, SD = 15.27) was significantly higher than that of NS.
Table 3.
Comparison of visual analog scale scores between different groups of smoking status
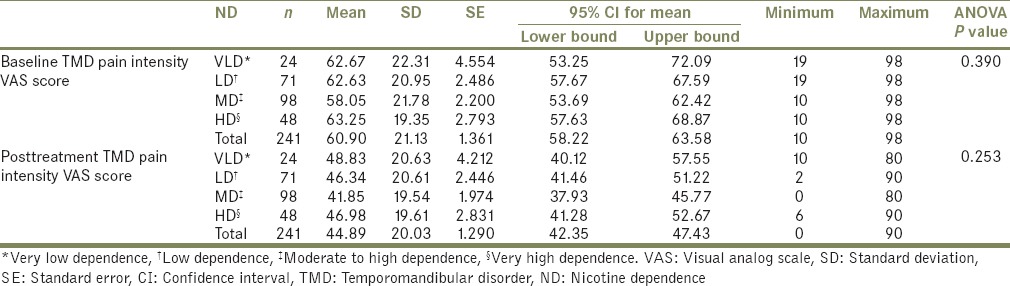
When the mean TMD pain intensity VAS scores between the four groups of ND were compared, no statistically significant differences were found at baseline (F[3237] =1.007, P = 0.390) or posttreatment (F[3237] =1.37, P = 0.253) [Table 4].
Table 4.
Comparison of visual analog scale scores between different groups of nicotine dependence
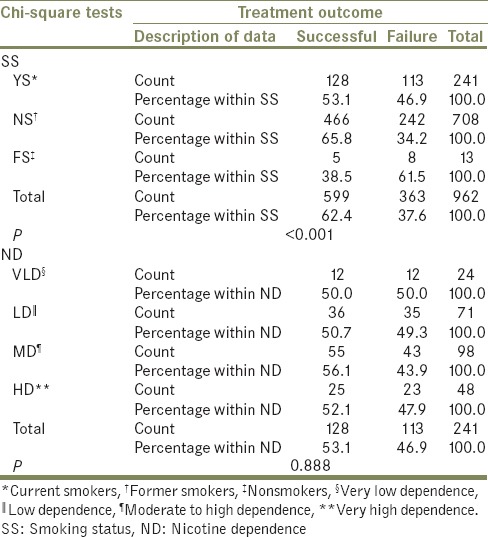
The majority of the patients in all three groups of SS showed a successful treatment outcome. The outcome of treatment was most successful in NS (65.8%) and least successful in FS (38.5%), with this difference being statistically significant (P ≤ 0.001). There were no significant differences within the groups of ND with respect to treatment outcome [P = 0.888, Table 5].
Table 5.
Comparison of treatment outcome among different groups of smoking status and nicotine dependence
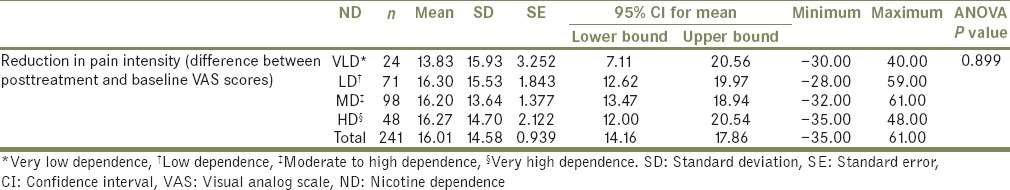
On comparing the mean reduction in pain intensity between different groups of SS, it was found that there was a statistically significant difference (F[2959] =7.14, P = 0.000) and was identified between YS (mean = 16.01, SD = 14.58) and NS (mean = 19.77, SD = 14.7) [Table 6]. No such significant difference in pain intensity reduction was found between the different groups of ND [F (3237) =0.197, P = 0.899, Table 7].
Table 6.
Comparison of reduction in pain intensity, between different groups of smoking status
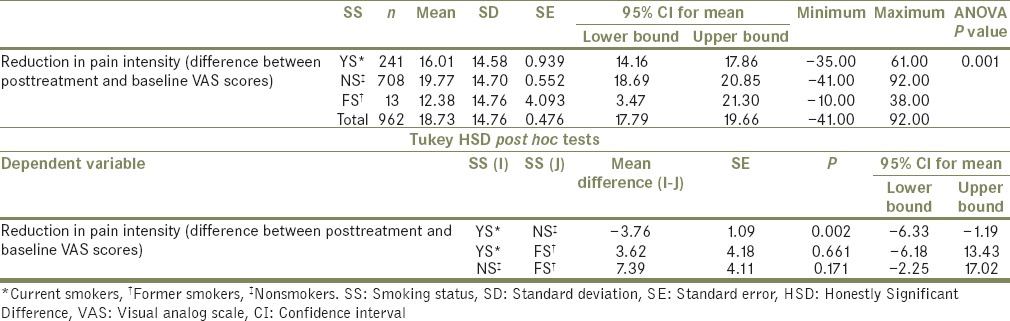
Table 7.
Comparison of reduction in pain intensity, between different groups of nicotine dependence
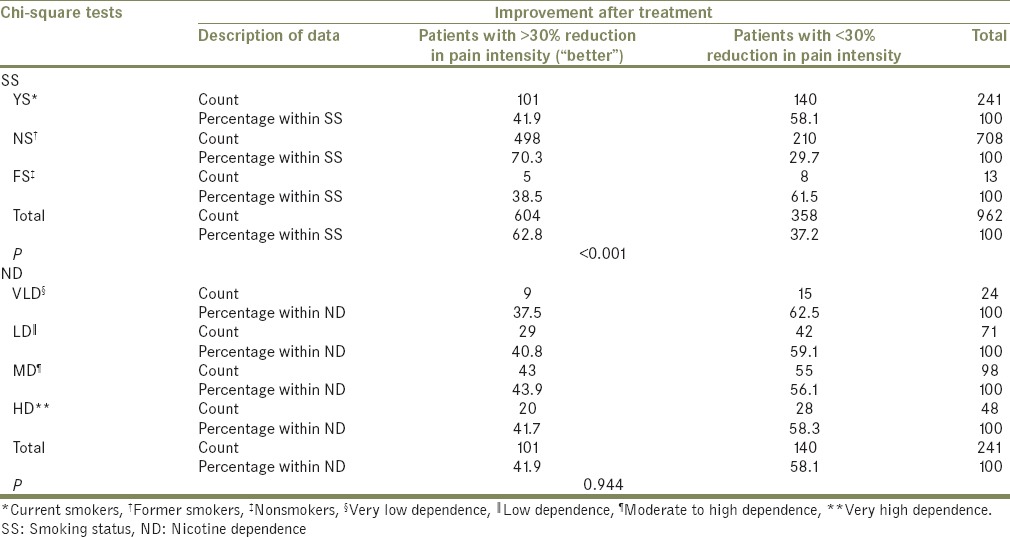
Out of the 962 patients, the number of patients who showed improvement after treatment (“better” patients with >30% reduction in TMD pain intensity) was 604 and differed significantly between the groups of SS (P ≤ 0.001), with most of the “better” patients being NS and the least being FS. There was no significant difference between groups of ND (P = 0.944) with respect to the number of patients who got better [Table 8].
Table 8.
Comparison of number of patients showing improvement (“better” patients) after treatment, among different groups of smoking status and nicotine dependence
DISCUSSION
There is little awareness about the possible association between smoking and TMD pain, despite epidemiologic and clinical evidence suggesting a link between tobacco use and pain of various forms.[9,10,11,12,13] The key finding in this study appears to be that YS show higher TMD pain intensity and respond less favorably to treatment than NS or FS. This outcome supports the theory that smoking increases pain and pain reporting.[68] Studies of different patient populations have also demonstrated that smoking is associated with greater pain intensity.[10,13,15,19] Yunus et al.'s study, which adjusted for education and age, found a positive relationship between smoking and pain in patients with fibromyalgia.[15] A study by Weingarten et al. found that pain intensity as measured by the graded chronic pain scale, a scale combining pain intensity with interference, was no longer significantly different between smoking and nonsmoking TMD patients.[11] Possible explanations for the conflicting results of the current study could be the smaller number of patients in Weingarten's study (about 600 patients, 15% of whom were smokers) and the use of a different pain intensity scale.
Results of the present study do not agree with Wanman's outcome either,[12] which showed similar signs and symptoms of TMD in YS and NS. This difference between the two studies may be attributed to the variation in sample size, and the fact that Wanman evaluated all signs and symptoms of TMD from Helkimo's anamnestic and dysfunction indices, instead of evaluating only the pain intensity.[69]
The relationship between SS and painful conditions is complicated by the fact that smoking can produce changes in the functioning of the central nervous system which persist long after patients stop smoking.[70] Thus, there may be a difference in the susceptibility to chronic pain between NS and FS, which was the case observed in this study. This study shows a statistically significant difference in pain intensity scores and reduction in pain intensity between NS and FS, but not between YS and FS. Several other studies also suggest that an association of smoking history and chronic pain conditions also exists among FS.[71,72,73]
The present study explored a dose-dependent relationship between smoking and pain intensity by taking into consideration the ND. The results showed no significant association between ND and pain intensity. This is similar to the outcome in Wanman's study.[12] In his study, when the smokers were divided into low-frequency smokers (n = 15), moderate-frequency smokers (n = 12), and high-frequency smokers (n = 11), no differences in TMD signs and symptoms were found among the groups. This lack of statistical significance of the difference between groups in his study was attributed to the small number of individuals included in each category.[12] In the present study, even though the dose-related groups were considerably larger in size (VLD-24, LD-71, MD-98, and HD-48), they did not differ in TMD pain intensity. However, there are studies with different results, in which higher dosages of nicotine substances were associated with higher odds of TMD[13] or more pronounced symptoms[19] in a dose–response relation, but the small number of YS in those studies reduced the precision of estimates making interpretation of the strength of the smoking and TMD relationship more difficult.
The global transition outcome measure in this study showed a significant difference in outcome between the groups of SS, with the NS being significantly more successful than YS or FS. The reduction in pain intensity and number of “better” patients was also significantly greater in NS as compared to YS. Taken together, this implies NS responded more favorably than YS or FS.
There are conflicting results regarding the association of smoking with the outcome of pain treatment. Few studies show that smokers presenting to pain treatment programs report more pain and greater functional impairment compared with NS.[11,16,19,22] Fishbain et al. found that YS were less likely to be employed compared with NS after multidisciplinary treatment for low back pain, implying more persistent disability.[14] Weingarten et al. observed that 50% of smokers presenting to an outpatient tertiary pain clinic were unemployed or disabled, compared with 18% of NS.[19] However, Hooten et al. reported that in an observational study of outcomes from a 3-week multidisciplinary pain rehabilitation program, despite the greater pain and functional impairment reported by smokers at program entry, their treatment responses were either not different or actually better than for NS.[21]
In the present study, the better treatment response in NS, when compared to YS, could be due to the fact that smoking has been associated with other comorbid conditions such as depression, anxiety, sleep disturbances, and bruxism, which may pose additional challenges to the treatment of smokers with painful symptoms. Another explanation can be sought from few studies that describe the relationship between smoking cigarettes as a mechanism to cope with chronic pain and pain-related outcome.[36] They state that pain may be a powerful reinforcer in the maintenance of tobacco smoking and ND. In the absence of more adaptive coping responses, persons with chronic pain may learn to rely on smoking to manage noxious internal states. Hence, smoking may contribute to exacerbation or maintenance of chronic pain, affecting the pain-related outcome of treatment.
FS was the only group with a majority of the patients showing unfavorable treatment response. FS comprised only 1.4% of the total study population, and this could have influenced the statistical outcome. It is also known that aryl hydrocarbon receptor (AhR) ligands have a long half-life, and it is possible that the lingering presence of AhR ligands within the body continue to mediate pain and cause functional interference.[70]
This study made use of the well-validated RDC for determining TMD case status. The effect of treatment was evaluated using a global transition outcome measure. This type of measure has limitations because it is a subjective assessment only. Verbal scales are categorical, making it difficult to specify the size of each category and whether the categories are of equal spacing. In other words, they are not very sensitive to changes in pain intensity. In anticipation of this, it was decided to include the VAS to evaluate pain after treatment. At the end of treatment, a minimum 30% reduction in pain compared to baseline, as measured using the VAS, was used as a criterion for improvement. This cutoff point was selected based on previous findings that approximately 70% of patients who had a 30% or more reduction in pain at rest, as measured with the VAS,[52] also agreed using global transition judgments that they were “better.”
All the patients were examined, treated, and evaluated by the same dentist, which eliminated the intra-examiner error and ensured that the examinations were made using the same protocol during the study. Unlike most similar studies which were done previously, this study was done on a relatively large sample size and investigated the possible dose-dependent relationships by taking into consideration the measures of ND.
There are, however, several limitations of the present study. Results from this study indicate that with respect to TMD pain intensity and outcome of pain treatment, FS did not differ significantly from NS, and there was no significant association with ND. The extremely small sample size of FS and greatly varied sample sizes of ND groups reduce the precision of estimates making interpretation of the former SS or ND and TMD relationship more difficult. The lack of details regarding time duration since the patients quit smoking is another factor that needs to be taken into consideration, with respect to FS.
The study evaluated the SS and the number of cigarettes smoked by the patients based on their self-report, but this information could be inaccurate especially in young people who are more likely to under-report their smoking habit. Other factors which could have affected the results of the current study were that the groups were not matched with each other in age and gender, psychological profile, the treatment they received, and probably differed on anamnestic and occlusal factors, presence of vicious habits and medication intake, which were not evaluated in the present study. The reason for not trying to match the samples was due to the large difference between the groups in terms of the number of patients included. Anxiety and psychological stress of the TMD patients were also not evaluated. Smokers with TMD may rely on tobacco to elevate mood and relieve comorbid depressive symptoms. This “reverse causation” might account for some of the observed association between smoking and TMD. The treatment modality that was chosen for this study varied between patients, from jaw exercises, splint therapy or both, depending upon the patient needs. This aspect of the study is a design flaw but unavoidable for ethical reasons. Further studies are needed to match the groups to control the mentioned variables and improve the quality of the study.
CONCLUSIONS
In this study, among Indian patients with TMD, smokers reported significantly greater pain intensity and poorer response to treatment than NS. This association was dose independent as ND had no significant effect on pain intensity or treatment outcome. Therefore, there is a need for health-care professionals to counsel smokers on the unfavorable impact of smoking on their pain condition and prognosis for improvement and to recommend smoking cessation as an integral part of the treatment plan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Meltzer EO. Prevalence, economic, and medical impact of tobacco smoking. Ann Allergy. 1994;73:381–9. [PubMed] [Google Scholar]
- 2.Sherman CB. Health effects of cigarette smoking. Clin Chest Med. 1991;12:643–58. [PubMed] [Google Scholar]
- 3.Albandar JM, Streckfus CF, Adesanya MR, Winn DM. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71:1874–81. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- 4.Bergström J, Eliasson S, Dock J. A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol. 2000;71:1338–47. doi: 10.1902/jop.2000.71.8.1338. [DOI] [PubMed] [Google Scholar]
- 5.Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL. Smoking and bone metabolism in elderly women. Bone. 2000;27:429–36. doi: 10.1016/s8756-3282(00)00341-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu XD, Zhu YK, Umino T, Spurzem JR, Romberger DJ, Wang H, et al. Cigarette smoke inhibits osteogenic differentiation and proliferation of human osteoprogenitor cells in monolayer and three-dimensional collagen gel culture. J Lab Clin Med. 2001;137:208–19. doi: 10.1067/mlc.2001.113066. [DOI] [PubMed] [Google Scholar]
- 7.Frymoyer JW, Pope MH, Costanza MC, Rosen JC, Goggin JE, Wilder DG. Epidemiologic studies of low-back pain. Spine (Phila Pa 1976) 1980;5:419–23. doi: 10.1097/00007632-198009000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Andersson H, Ejlertsson G, Leden I. Widespread musculoskeletal chronic pain associated with smoking. An epidemiological study in a general rural population. Scand J Rehabil Med. 1998;30:185–91. [PubMed] [Google Scholar]
- 9.Jay SJ. Tobacco use and chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:2398–2401. doi: 10.1001/archinte.160.15.2398. [DOI] [PubMed] [Google Scholar]
- 10.Melis M, Lobo SL, Ceneviz C, Ruparelia UN, Zawawi KH, Chandwani BP, et al. Effect of cigarette smoking on pain intensity of TMD patients: A pilot study. Cranio. 2010;28:187–92. doi: 10.1179/crn.2010.026. [DOI] [PubMed] [Google Scholar]
- 11.Weingarten TN, Iverson BC, Shi Y, Schroeder DR, Warner DO, Reid KI. Impact of tobacco use on the symptoms of painful temporomandibular joint disorders. Pain. 2009;147:67–71. doi: 10.1016/j.pain.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Wänman A. Temporomandibular disorders among smokers and nonsmokers: A longitudinal cohort study. J Orofac Pain. 2005;19:209–17. [PubMed] [Google Scholar]
- 13.Sanders AE, Maixner W, Nackley AG, Diatchenko L, By K, Miller VE, et al. Excess risk of temporomandibular disorder associated with cigarette smoking in young adults. J Pain. 2012;13:21–31. doi: 10.1016/j.jpain.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishbain DA, Lewis JE, Cole B, Cutler RB, Rosomoff HL, Rosomoff RS. Variables associated with current smoking status in chronic pain patients. Pain Med. 2007;8:301–11. doi: 10.1111/j.1526-4637.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- 15.Yunus MB, Arslan S, Aldag JC. Relationship between fibromyalgia features and smoking. Scand J Rheumatol. 2002;31:301–5. doi: 10.1080/030097402760375214. [DOI] [PubMed] [Google Scholar]
- 16.Weingarten TN, Podduturu VR, Hooten WM, Thompson JM, Luedtke CA, Oh TH. Impact of tobacco use in patients presenting to a multidisciplinary outpatient treatment program for fibromyalgia. Clin J Pain. 2009;25:39–43. doi: 10.1097/AJP.0b013e31817d105e. [DOI] [PubMed] [Google Scholar]
- 17.Pamuk ON, Dönmez S, Cakir N. The frequency of smoking in fibromyalgia patients and its association with symptoms. Rheumatol Int. 2009;29:1311–4. doi: 10.1007/s00296-009-0851-5. [DOI] [PubMed] [Google Scholar]
- 18.Logan HL, Fillingim RB, Bartoshuk LM, Sandow P, Tomar SL, Werning JW, et al. Smoking status and pain level among head and neck cancer patients. J Pain. 2010;11:528–34. doi: 10.1016/j.jpain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, Warner DO. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11:643–53. [PubMed] [Google Scholar]
- 20.De Leeuw R, Eisenlohr-Moul T, Bertrand P. The association of smoking status with sleep disturbance, psychological functioning, and pain severity in patients with temporomandibular disorders. J Orofac Pain. 2013;27:32–41. doi: 10.11607/jop.1040. [DOI] [PubMed] [Google Scholar]
- 21.Hooten WM, Townsend CO, Bruce BK, Schmidt JE, Kerkvliet JL, Patten CA, et al. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain Med. 2009;10:347–55. doi: 10.1111/j.1526-4637.2008.00494.x. [DOI] [PubMed] [Google Scholar]
- 22.Keil T, Lau S, Roll S, Gruber C, Nickel R, Niggemann B, et al. Maternal smoking increases risk of allergic sensitization and wheezing only in children with allergic predisposition: Longitudinal analysis from birth to 10 years. Allergy. 2009;64:445–51. doi: 10.1111/j.1398-9995.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 23.Koçer A, Koçer E, Memisogullari R, Domaç FM, Yüksel H. Interleukin-6 levels in tension headache patients. Clin J Pain. 2010;26:690–3. doi: 10.1097/AJP.0b013e3181e8d9b6. [DOI] [PubMed] [Google Scholar]
- 24.Taylor RG, Gross E, Joyce H, Holland F, Pride NB. Smoking, allergy, and the differential white blood cell count. Thorax. 1985;40:17–22. doi: 10.1136/thx.40.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaerøy H, Helle R, Førre O, Kåss E, Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: New features for diagnosis. Pain. 1988;32:21–6. doi: 10.1016/0304-3959(88)90019-X. [DOI] [PubMed] [Google Scholar]
- 26.del Arbol JL, Muñoz JR, Ojeda L, Cascales AL, Irles JR, Miranda MT, et al. Plasma concentrations of beta-endorphin in smokers who consume different numbers of cigarettes per day. Pharmacol Biochem Behav. 2000;67:25–8. doi: 10.1016/s0091-3057(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 27.Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–85. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Jamison RN, Stetson BA, Parris WC. The relationship between cigarette smoking and chronic low back pain. Addict Behav. 1991;16:103–10. doi: 10.1016/0306-4603(91)90002-y. [DOI] [PubMed] [Google Scholar]
- 29.Ernst E. Smoking, a cause of back trouble? Br J Rheumatol. 1993;32:239–42. doi: 10.1093/rheumatology/32.3.239. [DOI] [PubMed] [Google Scholar]
- 30.Líndal E, Stefánsson JG. Connection between smoking and back pain – Findings from an Icelandic general population study. Scand J Rehabil Med. 1996;28:33–8. [PubMed] [Google Scholar]
- 31.Battié MC, Videman T, Gill K, Moneta GB, Nyman R, Kaprio J, et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: An MRI study of identical twins. Spine (Phila Pa 1976) 1991;16:1015–21. [PubMed] [Google Scholar]
- 32.An HS, Silveri CP, Simpson JM, File P, Simmons C, Simeone FA, et al. Comparison of smoking habits between patients with surgically confirmed herniated lumbar and cervical disc disease and controls. J Spinal Disord. 1994;7:369–73. [PubMed] [Google Scholar]
- 33.Holm S, Nachemson A. Nutrition of the intervertebral disc: Acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93:91–9. doi: 10.1517/03009734000000042. [DOI] [PubMed] [Google Scholar]
- 34.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126:1131–4. doi: 10.1001/archsurg.1991.01410330093013. [DOI] [PubMed] [Google Scholar]
- 35.Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117:467–72. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, Morasco BJ. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. J Pain. 2012;13:285–92. doi: 10.1016/j.jpain.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. Tobacco smoking in India: Prevalence, quit-rates and respiratory morbidity. Indian J Chest Dis Allied Sci. 2006;48:37–42. [PubMed] [Google Scholar]
- 38.Rani M, Bonu S, Jha P, Nguyen SN, Jamjoum L. Tobacco use in India: Prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control. 2003;12:e4. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maizlin ZV, Nutiu N, Dent PB, Vos PM, Fenton DM, Kirby JM, et al. Displacement of the temporomandibular joint disk: Correlation between clinical findings and MRI characteristics. J Can Dent Assoc. 2010;76:a3. [PubMed] [Google Scholar]
- 40.Katyayan PA, Katyayan MK, Shah RJ, Patel G. Efficacy of appliance therapy on temporomandibular disorder related facial pain and mandibular mobility: A randomized controlled study. J Indian Prosthodont Soc. 2014;14:251–61. doi: 10.1007/s13191-013-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeResche L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 42.Shet RG, Rao S, Patel R, Suvvati P, Sadar LR, Yadav RD. Prevalence of temporomandibular joint dysfunction and its signs among the partially edentulous patients in a village of North Gujarat. J Contemp Dent Pract. 2013;14:1151–5. doi: 10.5005/jp-journals-10024-1466. [DOI] [PubMed] [Google Scholar]
- 43.Bagis B, Ayaz EA, Turgut S, Durkan R, Özcan M. Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: A retrospective study on 243 consecutive patients. Int J Med Sci. 2012;9:539–44. doi: 10.7150/ijms.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopal SK, Ram Shankar S, Vardhan BH. Prevalence of temporo-mandibular joint disorders in symptomatic and asymptomatic patients: A cross-sectional study. Int J Adv Health Sci. 2014;1:14–20. [Google Scholar]
- 45.Modi P, Shaikh SS, Munde A. A cross sectional study of prevalence of temporomandibular disorders in university students. Int J Sci Res Public. 2012;2:1–3. [Google Scholar]
- 46.Chaiton MO, Cohen JE, O’Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health. 2009;9:356. doi: 10.1186/1471-2458-9-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Croghan IT, Bronars C, Patten CA, Schroeder DR, Nirelli LM, Thomas JL, et al. Is smoking related to body image satisfaction, stress, and self-esteem in young adults? Am J Health Behav. 2006;30:322–33. doi: 10.5555/ajhb.2006.30.3.322. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: Findings from a 10-year longitudinal study. Addiction. 2010;105:1652–9. doi: 10.1111/j.1360-0443.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- 49.Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: A prospective study over 3 years. Am J Public Health. 1998;88:1518–22. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005-2008. NCHS Data Brief. 2010;34:1–8. [PubMed] [Google Scholar]
- 51.Lavigne GL, Lobbezoo F, Rompré PH, Nielsen TA, Montplaisir J. Cigarette smoking as a risk factor or an exacerbating factor for restless legs syndrome and sleep bruxism. Sleep. 1997;20:290–3. [PubMed] [Google Scholar]
- 52.Ahlberg J, Savolainen A, Rantala M, Lindholm H, Könönen M. Reported bruxism and biopsychosocial symptoms: A longitudinal study. Community Dent Oral Epidemiol. 2004;32:307–11. doi: 10.1111/j.1600-0528.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 53.Poveda Roda R, Bagan JV, Díaz Fernández JM, Hernández Bazán S, Jiménez Soriano Y. Review of temporomandibular joint pathology. Part I: Classification, epidemiology and risk factors. Med Oral Patol Oral Cir Bucal. 2007;12:E292–8. [PubMed] [Google Scholar]
- 54.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 55.Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–7. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 56.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 57.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 58.Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: A comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström test for nicotine dependence (FTND) in a clinical sample. Addict Behav. 1994;19:307–17. doi: 10.1016/0306-4603(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 59.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 60.De Leeuw R. Orofacial Pain: Guidelines for Assessment, Diagnosis and Management. 4th ed. Chicago, IL: Quintessence; 2008. American Academy of orofacial pain; pp. 129–75. [Google Scholar]
- 61.Okeson JP. Management of Temporomandibular Disorders and Occlusion. 7th ed. St. Louis, MO: Elsevier Health Sciences; 2008. pp. 258–399. [Google Scholar]
- 62.Yuasa H, Kino K, Kubota E, Kakudo K, Sugisaki M, Nishiyama A, et al. Primary treatment of temporomandibular disorders: The Japanese Society for the temporomandibular joint evidence-based clinical practice guidelines. Jpn Dent Sci Rev. 2013;49:89–98. [Google Scholar]
- 63.Hegde V. A review of the disorders of the temporomandibular joint. J Indian Prosthodont Soc. 2005;5:56–61. [Google Scholar]
- 64.Carlsson GE, Magnusson T. Management of Temporomandibular Disorders in the General Dental Practice. Chicago: Quintessence; 1999. pp. 93–103. [Google Scholar]
- 65.Okeson JP, Kemper JT, Moody PM. A study of the use of occlusion splints in the treatment of acute and chronic patients with craniomandibular disorders. J Prosthet Dent. 1982;48:708–12. doi: 10.1016/s0022-3913(82)80034-6. [DOI] [PubMed] [Google Scholar]
- 66.Dao TT, Lund JP, Lavigne GJ. Pain responses to experimental chewing in myofascial pain patients. J Dent Res. 1994;73:1163–7. doi: 10.1177/00220345940730060601. [DOI] [PubMed] [Google Scholar]
- 67.Grossi ML, Goldberg MB, Locker D, Tenenbaum HC. Reduced neuropsychologic measures as predictors of treatment outcome in patients with temporomandibular disorders. J Orofac Pain. 2001;15:329–39. [PubMed] [Google Scholar]
- 68.Brage S, Bjerkedal T. Musculoskeletal pain and smoking in Norway. J Epidemiol Community Health. 1996;50:166–9. doi: 10.1136/jech.50.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helkimo M. Studies on function and dysfunction of the masticatory system. II. Index for anamnestic and clinical dysfunction and occlusal state. Sven Tandlak Tidskr. 1974;67:101–21. [PubMed] [Google Scholar]
- 70.Perkins KA, Gerlach D, Broge M, Sanders M, Grobe J, Fonte C, et al. Quitting cigarette smoking produces minimal loss of chronic tolerance to nicotine. Psychopharmacology (Berl) 2001;158:7–17. doi: 10.1007/s002130100850. [DOI] [PubMed] [Google Scholar]
- 71.Palmer KT, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: Findings from a British National Survey. Ann Rheum Dis. 2003;62:33–6. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.John U, Hanke M, Meyer C, Völzke H, Baumeister SE, Alte D. Tobacco smoking in relation to pain in a national general population survey. Prev Med. 2006;43:477–81. doi: 10.1016/j.ypmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Jakobsson U. Tobacco use in relation to chronic pain: Results from a Swedish population survey. Pain Med. 2008;9:1091–7. doi: 10.1111/j.1526-4637.2008.00473.x. [DOI] [PubMed] [Google Scholar]


