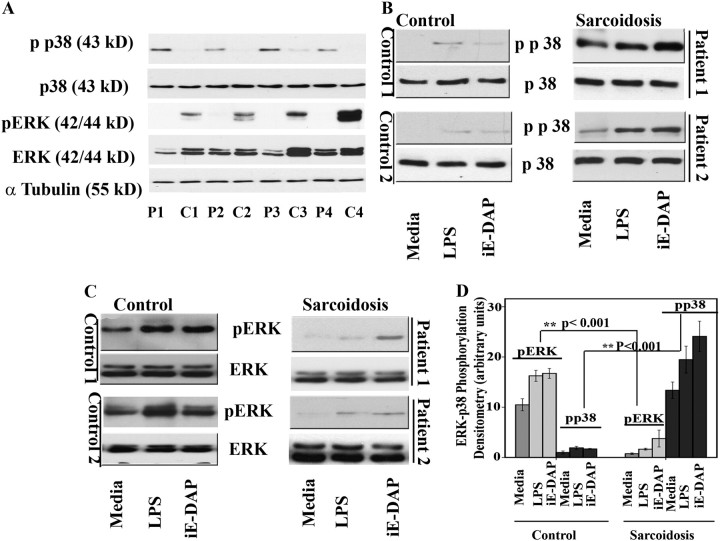
Figure 3.
Nucleotide-binding oligomerization domain 1 (NOD1) and Toll-like receptor 4 (TLR 4) stimulation leads to p38 and extracellular signal–regulated kinase (ERK) mitogen-activated protein (MAP) kinase phosphorylation in cultured human bronchoalveolar lavage (BAL) cells. Whole cell extracts were prepared from freshly obtained cells collected during BAL of control subjects and patients, and 30 μg of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis. (A) Whole cell lysates of four control subjects (C1–C4) and four patients (P1–P4). Blots were probed with antibodies against the phosphorylated forms of ERK and p38 as well as total ERK and total p38. Equal loading was confirmed using α-tubulin antibody. (B) BAL cells obtained from control subjects and patients were placed in culture immediately after isolation with and without γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 45 minutes. Phosphorylation of p38 in BAL cells of patients and control subjects was analyzed using whole cell lysates and specific antibodies against the phosphorylated form (Thr180/Tyr182) and total p38. Representative results are shown for two patients and two control subjects out of a total of n = 12 in each group. (C) Phosphorylation of ERK in BAL cells of patients and control subjects in response to LPS, iE-DAP, and a combination of both. Western blot analysis was performed using antibodies against the phosphorylated form of ERK (Thr202/Tyr204). Equal loading was determined using total ERK. (D) Densitometric values (mean ± SEM) of phosphorylated form of ERK and p38 of eight different experiments. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05; **P < 0.001. Significant differences were found in phosphorylated form of both p38 and ERK kinase in disease and control subjects.