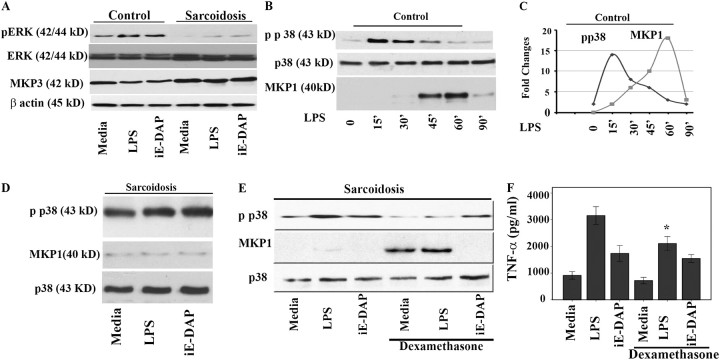
Figure 5.
Mitogen-activated protein kinase phosphate (MKP)-1 and MKP-3 expression in bronchoalveolar lavage (BAL) cells and healthy peripheral blood monocytes (PBMCs). (A) MKP-3 is down-regulated in response to nucleotide-binding oligomerization domain–like receptor (NLR) and Toll-like receptor 4 (TLR 4) ligands. BAL cells (5 × 107 cells) obtained from patients with sarcoidosis and control subjects were placed in culture immediately after isolation and treated with γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) (1 μg/ml) or LPS (1 ng/ml) for 45 minutes or left in media without any treatment (control). Whole cell lysates were subjected to Western blot analysis using antibodies recognizing the active phosphorylated form of extracellular signal–regulated kinase (ERK) and a polyclonal antibody against human MKP-3. Equal loading of protein was confirmed using total ERK or β-actin antibodies. Patients demonstrated higher basal levels of MKP3 compared with control subjects. Both groups responded with a down-regulation of MKP3 after nucleotide-binding oligomerization domain 1 (NOD1) and LPS treatment, with patients responding to a lesser degree. Three other treatments with different ligands were cut from the original blot and excluded from the present image. Representative Western blot analyses from six separate experiments (patients) are shown. (B) PBMCs of healthy volunteers were isolated and cells were cultured to a density of 5 × 107 per well and treated with 100 ng/ml LPS for different periods of time. Total cell lysates from human monocytes (n = 3) were subjected to Western blot analysis using antibodies specific for the phosphorylated form of p38 (Thr180/Tyr182), total p38, and MKP-1. Two lanes (treated with different ligands) from the image for the total p38 and phospho p38 were excluded. (C) Dynamic relation of p38 and MKP-1 in isolated PBMC of healthy subjects. Densitometric quantifications from Western blots were performed from three separate experiments for phospho p38 and MKP-1 in response to LPS. The intensity of bands for phospho p38 and MKP1 are plotted against time. (D) Lack of MKP-1 induction in BAL cells of patients with sarcoidosis. BAL cells (5 × 107 cells) obtained from patients with sarcoidosis (n = 12) were placed in culture and treated with iE-DAP (1 μg/ml) or LPS (1 ng/ml) for 60 minutes or left in media without any treatment (media). Whole cell lysates were subjected to Western blot analyses using antibodies specific to phospho p38 (upper panel), MKP-1, and total p38 (as control). (E) Dexamethasone induces MKP-1 in BAL cells. Cells of patients (n = 3) were pretreated with dexamethasone (100 ng/ml) for 45 minutes or kept in media. The cells were treated with NOD1 or TLR4 ligands as indicated. Immunoblotting was performed using phospho p38 (Thr180/Tyr182), and MKP1 antibodies. Equal loading was determined using total p38 antibody. This is a representative Western blot from three different experiments. (F) BAL cells obtained from patients were counted and placed in culture immediately after isolation. Cells were pretreated either with 100 ng/ml dexamethasone for 45 minutes before stimulation with iE-DAP (1 μg/mL) or LPS (1 ng/mL) for 16 hours. Tumor necrosis factor (TNF)-α immunoreactivity in supernatants was analyzed by ELISA. Using analysis of variance Mann-Whitney U test P < 0.05 was considered significant. *P < 0.05.