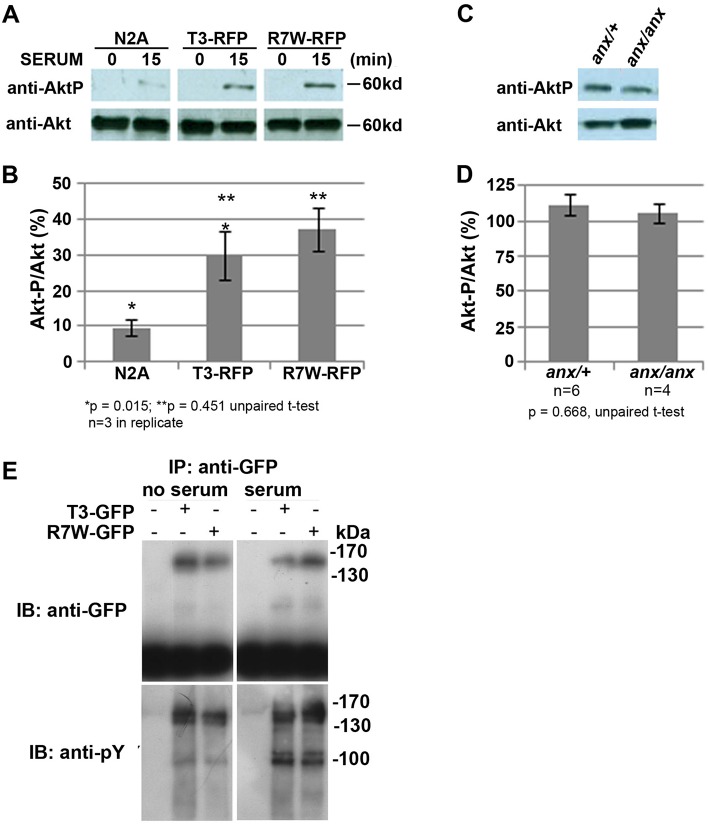
Fig. 6.
R7W-Tyro3 and Tyro3 affect Akt phosphorylation indistinguishably in cell culture and in anx/anx cerebella. Stable transfection of R7W-Tyro3 and Tyro3 results in equivalent Akt phosphorylation in response to serum. (A) Phosphorylation of Akt in response to 15 min exposure to serum after 12 h serum starvation in N2A cells stably expressing equivalent amounts of either Tyro3-RFP or R7W-Tyro3-RFP. (B) Percentage of phosphorylated Akt in response to serum. (C,D) Akt is phosphorylated to a similar extent in anx/anx and anx/+ cerebella. (C) Western blot detects equivalent levels of Akt-P in cerebella of anx/+ and anx/anx P10 mice. (D) The percentage of phosphorylated to unphosphorylated Akt is shown. (E) Serum-activated tyrosine phosphorylation of Tyro3-GFP and R7W-Tyro3-GFP seems equivalent in transiently transfected COS7 cells. In the absence and presence of serum, immunoprecipitation of Tyro3-GFP and R7W-Tyro3-GFP was carried out with an anti-GFP antibody. Subsequent analysis with an anti-phosphotyrosine antibody revealed no differences in tyrosine phosphorylation as indicated by equivocal banding patterns. *P<0.05, **P<0.01 by unparied t-test; data represented as mean±s.e.m.