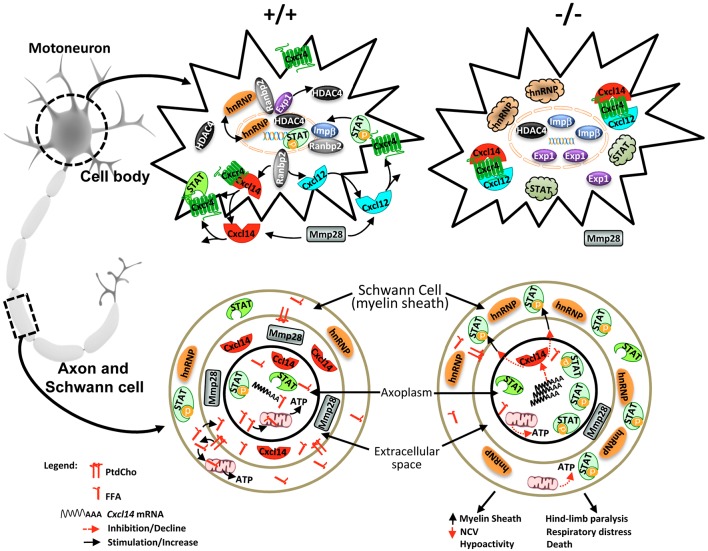
Fig. 10.
Mechanistic model of Ranbp2 functions in motoneuron and disease. In cell bodies of motoneurons of wild-type mice (upper left), Ranbp2 localizes at the cytosolic face of the nuclear pore complex, where it couples with the nuclear import and export receptors importin β and exportin 1, respectively, and controls rate-limiting steps of nucleocytoplasmic shuttling of these receptors and substrates, such as HDAC4, hnRNPH3 and Stat3. The biogenesis and secretion of Cxcl14 and Cxcl12 are dependent on their receptor, Cxcr4, and on Ranbp2. Cxcl14 and Cxcl12 are inhibitory and stimulatory ligands of Cxcr4, the activation of which promotes Stat3 phosphorylation (P-Stat3) and nuclear import of P-Stat3 by importin β. Metalloproteinase 28 (Mmp28) is secreted from motoneurons and controls the homeostasis of chemokine signaling. Loss of Ranbp2 in cell bodies causes the uncoupling of crucial steps by the nuclear import and export receptors, and substrates thereof. This uncoupling leads to the nuclear retention of importin β and HDAC4, redistribution of exportin 1 between the nuclear and cytosolic compartments, and formation of cytoplasmic inclusions of Cxcl14-Cxcl12-Cxcr4 complexes, and latent and activated Stat3 (upper right). In motoneuronal axons of wild-type mice (lower left), there is free fatty acid oxidation by the mitochondria and liberation of fatty acids from PthCho. hnRNPH3 is localized to Schwann cells but is absent from the axoplasm. Cxcl14 likely exerts paracrine neuroglial signaling activities in concert with activated Stat3 and Mmp28. Loss of Ranbp2 promotes strong declines in PthCho and FFA. These declines lower ATP production from decreased FFA oxidation (bottom right). There is also an accumulation of Cxcl14 mRNA, suppression of Cxcl14 synthesis and inhibitory signaling, and a decrease of Mmp28 levels caused by potential deficits in axoplasmic trafficking that lead to the paracrine upregulation of hnRNPH3 and activation of Stat3 in Schwann glia. Collectively, the dysregulations of these Ranbp2-dependent molecular processes promote the increase of the thickness of the axonal myelin sheath (g-ratio), the decrease of NCV and hypoactivity followed by hindlimb paralysis, respiratory distress and death of mice. PthCho, phosphatidylcholine; FFA, free fatty acid; hnRNP, hnRNPH3.