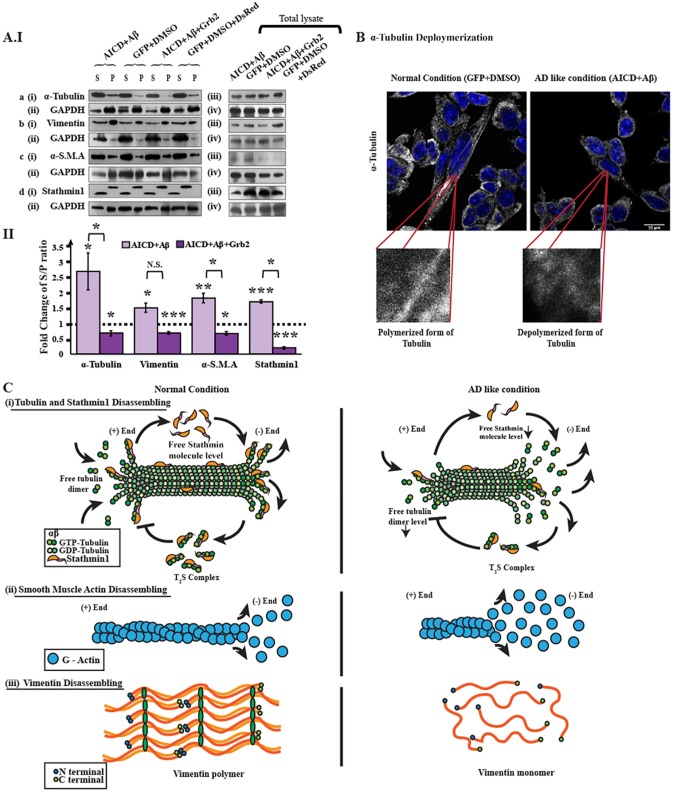
Fig. 3.
Disassembly assay of cytoskeletal proteins. The microtubule network comprises α- and β-tubulin monomers, and many microtubule-associated proteins (MAPs). Stathmin1 is one such MAP. Similarly, actin polymers are also composed of globular actin (G-Actin) monomers. As α-smooth muscle actin (α-SMA) is nothing but a variant of actin, it is disassembled in a manner similar to that of general actin polymers. Vimentin is a stack of aggregated vimentin peptide monomers, which have a distinct pattern. (AIa) Western blots represent (i) α-tubulin in the supernatant and pellet fractions for disease-inducing (AICD and Aβ) and reversal (AICD, Aβ and Grb2) conditions, along with (ii) an internal control GAPDH. (iii),(iv) Alterations of α-tubulin in total cell lysate. Western blots for other three cytoskeletal proteins – vimentin, α-SMA and stathmin 1 – are also depicted in AIb(i-iv), AIc(i-iv), AId(i-iv), respectively. (AII) Bar diagram demonstrating the alterations in disassembling factors (S/P ratio) for α-tubulin, vimentin, α-SMA and stathmin 1. Values are presented as the fold change relative to negative controls (empty vector or the solvent used for reagents). (B) Immunocytochemistry result depicting disassembly of α-tubulin polymer tracks in AICD-transfected and Aβ-treated cells. α-tubulin is shown in grey, and the nucleus is marked with DAPI staining. Zoomed images of polymer tracks for both conditions are given in the inset box. (C) Cartoon representations of molecular events of disassembly of (i) tubulin and stathmin 1, (ii) α-smooth muscle actin (α-SMA) and (iii) vimentin, respectively.