Abstract
Molecular oxygen (O2) displays very interesting properties. Its first excited state, commonly known as singlet oxygen (1O2), is one of the so-called Reactive Oxygen Species (ROS). It has been implicated in many redox processes in biological systems. For many decades its role has been that of a deleterious chemical species, although very positive clinical applications in the Photodynamic Therapy of cancer (PDT) have been reported. More recently, many ROS, and also 1O2, are in the spotlight because of their role in physiological signaling, like cell proliferation or tissue regeneration. However, there are methodological shortcomings to properly assess the role of 1O2 in redox biology with classical generation procedures. In this review the direct optical excitation of O2 to produce 1O2 will be introduced, in order to present its main advantages and drawbacks for biological studies. This photonic approach can provide with many interesting possibilities to understand and put to use ROS in redox signaling and in the biomedical field.
Keywords: Singlet oxygen, Reactive Oxygen Species (ROS), Redox biology, Biophotonics, Low-level laser therapy (LLLT), Photodynamic therapy (PDT)
Graphical abstract
Highlights
-
•
Singlet oxygen can be produced by direct optical excitation of molecular oxygen.
-
•
The wavelengths involved cover the infrared and visible spectra.
-
•
Very high spatial-temporal control over ROS-production in biological systems.
-
•
It offers robust experimental control for redox biology research.
1. Introduction
As presented in the enlightening and seminal book “Oxygen: The molecule that made the world” by Lane [1], molecular oxygen (O2) is a fascinating compound. This element has shaped the Earth at many levels, from the geophysical one to the atmosphere and living entities. Its build-up in the atmosphere around 2.7-2.3 billion years ago triggered what has probably been the most devastating biological crisis and extinction event the Earth has witnessed. Once oxygen had accumulated in sufficient quantities, living organisms adapted to it, some of them even “learning” how to use it to further oxidize reduced compounds to obtain much more energy.
With this oxygen incorporation to the metabolic network of living cells, it came as no surprise that oxygen, and its partly reduced chemical products known as Reactive Oxygen Species (ROS), also assumed regulating and signaling roles [2]. This signaling role has only recently started to be acknowledged by the scientific community, in what is becoming a new branch of biology known as Redox Biology [3], [4], [5]. Within this field, the deleterious effects of ROS, whenever they are produced in too large quantities or at inappropriate times/places in living organisms, are also explored to provide answers to many disorders and diseases that have their origin in a redox deregulation of cell physiology.
Currently, the physiological and pathological role of ROS is a very fast growing area. In this review I will focus in one of these ROS, in particular singlet oxygen (1O2). Even within this reactive species, a whole range of articles and reviews already exist ([6], [7], [8], [9], [10] to name a few reviews), as it is usually invoked as the main cytotoxic compound involved in the clinical therapeutic approach known as Photodynamic Therapy (PDT) [11], [12], [13], [14], [15]. However, it has turned out that 1O2, as it happens with many other ROS, is also involved in physiological cellular processes [2], [16], [17], [18], and/or modulates the cellular redox potential in such a way as to induce those processes [19], [20], [21]. As such, the study of 1O2 both as a biological damaging compound as well as a cellular signaling agent is of utmost importance. Classically, 1O2 has been generated in living systems employing a photodynamic approach (see Section 2.2 for more details on this). Recently, however several groups have re-sparked interest on the possibility to directly excite the oxygen molecule with light at certain wavelengths, for biological research or clinical applications [22]. Some successful light wavelengths employed towards this end have been 1270 nm [23], [24], [25], [26], [27], [28], 1065 nm [29], [30], [31] and 760 nm [32], [33], [34], to name a few. However, it must be mentioned (see details in Section 4) that research on the direct optical excitation of O2 predates by several decades these studies.
In this review I will present and elaborate on the different optical wavelength regions to directly excite O2 with light. First, I will briefly introduce the excited states of molecular oxygen, and their classical excitation approaches (Section 2). Then, an introduction to the biological action of 1O2 will be provided (Section 3). In Section 4 the available spectral regions to directly excite O2 for biological purposes will be detailed, with emphasis on their advantages and drawbacks. Finally, an outlook on different established or currently under development research areas that can take advantage of this photonic approach will be delineated (Section 5). It must be stressed that this review will deal with the available photonic options to excite O2, with redox biology studies in mind. It will only tangentially deal with the proper redox biology and chemistry of 1O2 (many different and excellent reviews have already been published on these topics and they will be cited whenever appropriate).
2. Singlet oxygen
The oxygen molecule O2 displays some very particular features. Being the second most electronegative element in the periodic table, it would be expected that O2 is to be a very reactive chemical compound. As it turns out, however, it is a quite unreactive molecule. A testimony to this is the fact that we live in a planet with a 21% oxygen atmospheric content, and nevertheless life thrives and even depends on it. Thus, I will briefly introduce the special characteristics of O2. From this starting point it will be quite straightforward to understand how O2 becomes electronically excited and, by extension, which are the main features of the first excited state commonly known as singlet oxygen (1O2), the topic of this review.
2.1. Basic physical-chemical features of 1O2
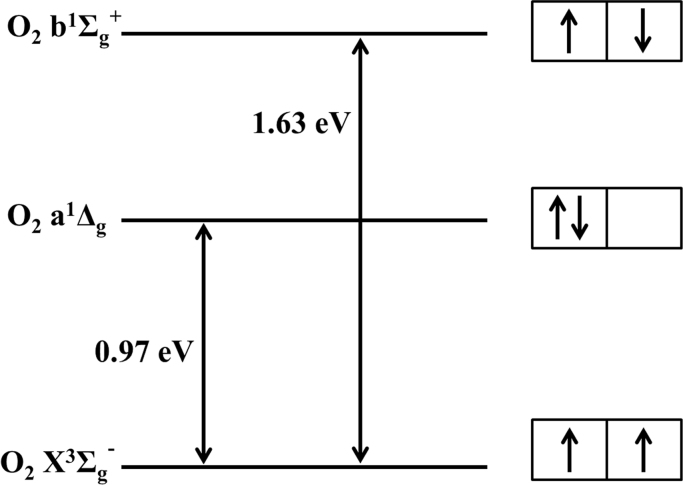
The reason why O2 is so unreactive, in spite of its electronegativity, is the fact that in the fundamental state it assumes a triplet electronic configuration [8], [9], [10], [35], [36]. The way in which the valence electrons arrange in O2 is such that the configuration with the least energy, has the two outermost electrons in different antibonding orbitals, but with the same spin (see Fig. 1). According to the molecular orbital theory, this arrangement has a triplet character. In formal spectroscopic nomenclature the ground state of O2 is designed as O2 X3Σg- [10], [35]. A less formal designation is 3O2, stressing the triplet character of the ground state. This ground state has a biradical chemical character, which makes it quite unreactive to most chemical compounds [35], [36]. At the same time, this triplet character makes ground state O2 a paramagnetic compound, where oxygen tends to move to the point of strongest magnetic field.
Fig. 1.
Electronic states of the oxygen molecule. The fundamental (O2 X3Σg-) and the two lowest electronic excited states of molecular oxygen (O2 a1Δg and O2 b1Σg+) are shown in the scheme. The fundamental, or ground, state is a triplet, while the two excited levels are singlets. The energy gap between the fundamental level and the other two levels is shown in eV. To the right, a simplified scheme of the different electronic arrangement featured by each electronic level. The boxes represent electronic orbitals, and the arrows the electronic spins.
Increasing in energy, the next two electronic levels of O2 are of singlet character. This is due to the rearrangement of the electron spin and orbital occupancy (Fig. 1). A certain amount of energy (~ 0.96 eV) is needed to flip one of the electron´s spin. Now, according to selection rules, both electrons can occupy the same orbital by coupling their spins. This first excited O2 level is designed as O2 (a1Δg), 1O2 (a) or simply 1O2 [10], [35]. A further increase in energy, to 1.62 eV above the ground level, maintains opposite electronic spins, thus singlet in character, but the two electrons again occupy different orbitals. This more energetic state is known as O2 (b1Σg+) or 1O2 (b) [10], [35], and it is also a singlet oxygen state. To simplify the nomenclature, and given the biological focus of this review, from this point on I will refer to ground state oxygen (O2 X3Σg-) simply as 3O2, the first singlet excited state -O2 (a1Δg)- as 1O2, and to the second singlet excited state - O2 (b1Σg+)- as 1O2 (b). The second excited state 1O2 (b) converts very fast (~ picoseconds) to the first excited state 1O2 [6], [37], especially in condensed matter (e.g. aqueous solutions), by transforming part of its electronic excitation energy into vibrational oscillations. Therefore, for most singlet oxygen applications in biology, the production of 1O2 (b) can be interpreted as if 1O2 had been directly produced instead. Many optical excitation bands that will be introduced later in this review (Section 4) produce 1O2 (b) in the first place, but this is very quickly transformed into 1O2 in biological systems.
Once produced 1O2 is several orders of magnitude more reactive than 3O2. This is a consequence of the molecule now having a singlet character, which makes it possible for it to interact more efficiently with most chemical compounds [2], [36], [38], [39], [40]. Most commonly, 1O2 reacts with other compounds by producing endoperoxides in the first place. These can further lead to generation of radicals, ROS, peroxides, aldehydes, etc. Most previous biological studies, studying the interaction between 1O2 and biological substrates, have focused on the deleterious reactions that 1O2 drives, which upset the smooth biochemistry of living systems. As it will be presented below, a new paradigm is emerging in which 1O2 can, under the right conditions, regulate physiological cell functions [16], [21], [33], [41], [42], [43]. Some of the prevalent reactions of 1O2 with biological substrates will be described in Section 3.
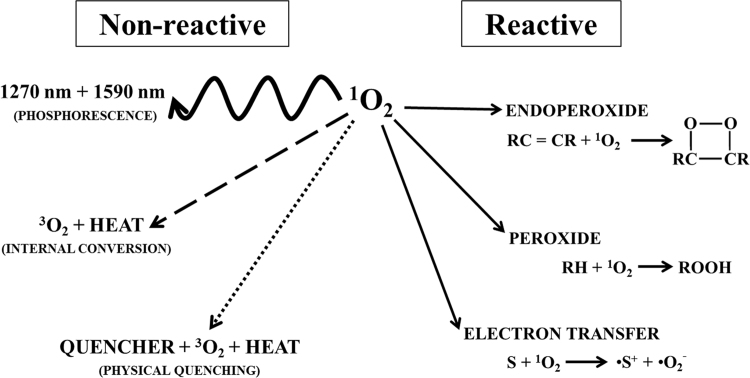
An important feature of 1O2 to consider in biological studies is its lifetime. This is the time it takes for a certain amount of 1O2 to decay to some fraction of its original concentration. As an excited species, 1O2 is in a metastable state: it decays to a lower energy state sooner or later. This decay can occur through different deactivation channels (Fig. 2). The simplest deactivation channel is for 1O2 to emit a photon and reach the fundamental state. As the initial and final electronic states are of different multiplicity (singlet vs. triplet), the light emission is termed phosphorescence. The emission is in a relatively narrow band centered at 1270 nm [6], [35], [36]. This radiative transition is forbidden by several electromagnetic and quantum mechanical rules [8]. As such, it takes a long time for it to happen in an undisturbed 1O2 molecule. However, in the condensed phase, the deactivation probability increases due to interaction and perturbations by nearby molecules or atoms, and the lifetime is reduced orders of magnitude. This 1270 nm emission is the counterpart of one of the absorption bands of 3O2 that are the main topic of this review (see Section 4.2).
Fig. 2.
Radiative and non-radiative deactivation pathways of 1O2. The excited 1O2 can undergo deactivation through different pathways, non-reactive (left) and reactive (right). It can experience a radiative transition to the ground state, emitting a phosphorescent photon at 1270 nm or 1590 nm. It can transform the electronic excitation energy directly to vibration and, eventually, heat. This can happen without external influence (internal conversion) or through environmental interactions with other compounds (physical quenching), like a solvent (e.g. H2O). 1O2 can engage in chemical reactions (reactive branch). Three prototypical kinds of reactions are shown: endoperoxide formation (top), peroxide formation (middle), and electron transfer (bottom). In the scheme R: chemical substituent, C: carbon atom, H: hydrogen atom, S: reduced substrate.
The main deactivation pathway in water and aqueous solutions is physical quenching by internal conversion to the ground state (Fig. 2). This non-radiative transition takes place spontaneously, competing with light emission at 1270 nm. In condensed media, its occurrence is greatly enhanced due to solvent or solute interaction [44]. The key aspect is that 1O2 deactivation takes place without a net chemical reaction. The excitation energy is lost to the medium first as vibrational energy, which finally decays to heat. The energy coupling among 1O2 and apolar non-hydrogenated solvents is much less efficient, and its lifetime is consequently prolonged, reaching very high values (milliseconds) [44], [45], [46]. Finally, 1O2 can engage in chemical reactions with a host of substrates. Some important examples directly related to biochemical and biological systems will be presented in Section 3.1.
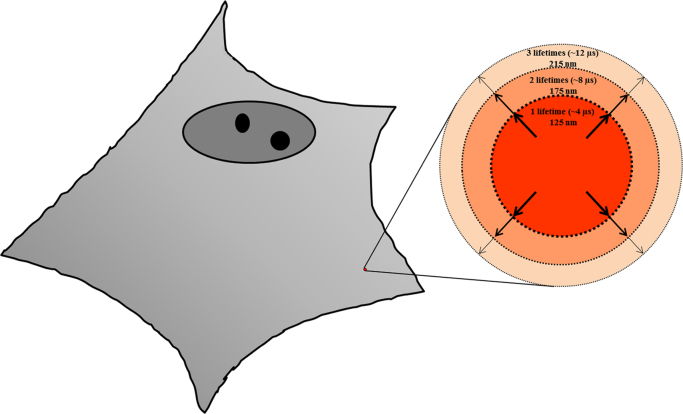
Usually the lifetime is expressed as the time it takes for a given 1O2 population to decay by 1/e (to ~36.78% of the initial amount) [47], [48]. The lifetime is highly variable (from 10−6 s to 103 s), and depends on different environmental parameters, like solvent, solvent phase (gas vs. condensed), temperature, polarity, hydrophobicity, etc [6]. Water happens to be one of the less “1O2-friendly” solvents. This is due to the very high coupling among the electronic excitation of 1O2 and the vibrational levels of the H2O molecule. In consequence, the most probable deactivation pathway for 1O2 in H2O is vibrational dissipation of energy, which quickly decays to heat [6], [48]. In aqueous solutions, which obviously are the medium of interest in biological systems, 1O2 lifetime is ~4 µs (Fig. 3) [47], [48]. This relatively short lifetime allows 1O2 to diffuse only a small distance from its inception spot. This diffusion distance has been calculated as ~100–150 nm during one lifetime [47], [48]. Thus, we would expect a relatively large spatial control over the “sphere of biological action” of 1O2 if we can produce 1O2 in a small enough volume. This topic will be elaborated further in Section 5.1. As ubiquitous as H2O is in living cells, some structures and organelles have a different composition (e.g. lipid membranes). As a consequence, 3O2 concentration and 1O2 lifetime can be considerably enhanced in those regions and, by extension, chemical/biological activity. These are parameters to be taken into account when 1O2 is generated in or close to those structures [34].
Fig. 3.
Diffusion distance of 1O2 in a cell. A prototypical eukaryotic adherent cell is shown to the left. A certain amount of 1O2 is initially produced in the indicated spot. Once produced, 1O2 can diffuse a certain distance before being deactivated by one of the mechanisms presented in Fig. 2. Taken together, they combine to limit the distance 1O2 can diffuse, which is displayed in the magnified circles to the right. The diffusion distances are correlated with the time it takes for oxygen to cover those (lifetimes). Therefore, if 1O2 is carefully produced within a constrained subcellular location (e.g. by a laser beam), it will initially perturb only a small cellular volume, offering interesting opportunities to assess biological action mechanisms. (Lifetimes and diffusion distances adapted from [48]).
A procedure to extend 1O2 lifetime and its activity sphere is to introduce heavy water (D2O) in a living system. D2O vibrational levels are slightly different from those of H2O. This is, nevertheless, enough to reduce energy transfer from 1O2 to D2O vibrational levels. As a result, 1O2 lifetime in pure D2O increases to ~68 µs and diffusion distance to ~500–600 nm [47], [48]. On the other hand, D2O is toxic for cells in the long term, but a compromise concentration of 25% − 50% D2O in H2O can be employed in experiments, which preserve cell viability enough to obtain relevant results. In fact, the use of D2O is a reliable method to prove 1O2 involvement in biological responses, as it will be explained in more detail in Section 5.1.
2.2. Classical excitation mechanisms of 1O2
The classical mechanisms of 1O2 excitation will now briefly be presented. In this way, the reader will be able to better appreciate the advantages and drawbacks of the direct optical excitation of 1O2, to be introduced in Section 4. One of the most common approaches is to employ a compound that transfers electronic excitation energy to 3O2. This compound is known as a photosensitizer (PS). When a PS is excited with light, it becomes electronically excited and can transfer this energy to the 3O2 molecule, under the right circumstances. It would be too lengthy to go into detail about this process, as it is outside the scope of this review. However, the interested reader is directed to some comprehensive publications in the literature on this topic [6], [7], [8], [9], [10], [12]. Suffice it to say that commonly employed PSs absorb light, from the near ultraviolet (near-UV) through the visible to the near infrared (NIR). These compounds show a relatively long excited state lifetime, which allows them to efficiently interact with 3O2 and transfer their energy to it. However, many PSs have drawbacks in greater or lesser degree when it comes to their incorporation or interaction with living systems (poor uptake, undesirable cell localization, induction of cell stress or straight cytotoxicity, etc.). For certain studies and applications, it can be desirable to excite 3O2 in other ways.
Several methodologies, variants of the classical light-PS photosensitized generation of 1O2, are currently in different developmental stages to deal with some of these drawbacks. The use of genetically-encoded PSs is gaining widespread use. In this approach cells are genetically-directed to synthesize a protein that harbors a PS (modified GFP or other proteins like the recently reported miniSOG) [49]. Under light excitation, these proteins generate ROS, one of them 1O2. This variant offers a large amount of control as to the subcellular PS localization [33], [34]. Given the short intracellular range of 1O2 mentioned in the previous paragraph, the precise positioning of a protein-PS within a certain cellular structure provides a great deal of control in regards to the biology of ROS. Among its drawbacks are the non-negligible interference with endogenous DNA, RNA and protein networks, the limited control over the precise protein-PS localization (for the moment), and the production of different ROS, not restricted to 1O2, during irradiation [33], [34].
A second variant to increase control over 1O2 production is the use of non-linear excitation of PSs. It also makes use of a conventional PS, which often has been selected according to its high two-photon absorption. By increasing the exciting light flux rate over the studied area, employing pulsed lasers, the PS absorbs two long-wavelength photons, whose combined energies allow the PS to reach an excited electronic state [10]. Once this happens, the usual energy transfer to 3O2 can take place, leading to 1O2 generation. Two-photon excitation depends critically on the light flux rate, and as a consequence only a focused spot of light is available for PS non-linear excitation. This leads to a very high spatial resolution for the 1O2 source spot (< 1 µm), which is certainly an asset in experiments done at the single cell level [33], [34]. However, its application is very limited when it comes to a larger target (e.g. organ or whole animal), and it usually happens that good two-photon absorbing PSs have other undesirable biological constrains, like inadequate cellular uptake. A very recent development of non-linear absorption-sensitized 1O2 generation is the pulsed excitation of metal or semiconductor nanoparticles, leading to the so-called hot-electron excitation [50], [51]. Briefly, a very short laser pulse (femtoseconds) excites a small population of electrons in the nanoparticle by plasmon resonance. These excited electrons gain a fairly large amount of energy (few eV). Before they thermalize (100 fs-1ps), they can engage in high-energy chemistry with compounds adsorbed at the nanoparticle´s surface, like 3O2 molecules, for example [52]. So, 1O2 is generated at the interface between nanoparticle and medium. As fascinating as this approach is, it is necessary to introduce the nanoparticles into the living system under study. And then, a fs-pulsed laser source is mandatory for the excitation mechanism to work. These considerations make this methodology useful only for certain experimental conditions, and probably will not become a widespread technique to study the biological role of 1O2.
A quite successful 1O2 production approach is the use of dark chemical reactions, in which light is unnecessary to achieve oxygen excitation. This is possible because certain highly exothermic reactions make enough free energy available to efficiently excite 3O2 [35], [36], [40]. The reaction produces some excited state product, that collides with 3O2 and transfers the excitation energy, after which 1O2 is obtained. Unfortunately, most chemical reactions producing 1O2 either require very reactive reagents and/or produce very reactive intermediates (e.g. classical reaction between hydrogen peroxide and hypochlorite). These chemical compounds are commonly incompatible with living systems, as they directly damage them. It is worth mentioning, however, that certain biochemical systems [17], [53] and cells [17], [54], [55] do indeed take advantage of some dark reactions to produce 1O2 without need of light. If this production has relevant biological roles, as it seems to, or they occur as a side effect and have a generic deleterious outcome, is not yet clear [2], [17]. More details on this will be provided in Section 3.
A particular class of chemical 1O2 sources deserves mentioning at this point. These are endoperoxides of organic molecules that form when the organic precursors interact with 1O2 [35], [56], [57]. The 1O2 becomes “stored” in the endoperoxide in a kind of latent state. Later, when these endoperoxides are exposed to higher temperatures or light, they release the 1O2 which then diffuses into the surroundings. By modulating the releasing parameter (commonly increasing the temperature), the biological action of 1O2 can be studied [16], [58], [59]. Advantages of this methodology are the relatively sharp control of the total amount of 1O2 released and the certainty that the only ROS generated in the first place is 1O2. A big issue can be the uptake/interaction of the endoperoxide with cells, and the cytotoxicity of these compounds per se (these organic compounds show aromatic portions which can, for example, lead to nucleic acid intercalation). All in all, they are a nice approach to the study of the biological impact of 1O2, although few publications report on their use towards this goal.
3. Redox biology of 1O2
Here, a brief introduction to the basic chemistry and biological action of 1O2 will be presented. The aim is to sketch the current knowledge in this area, so that the advantages and opportunities, as well as some of the drawbacks, which direct optical excitation of 1O2 offers, can be better understood and appreciated.
3.1. Redox chemistry
The chemistry of 1O2 is dominated, as would be expected, by a strong oxidative behavior. Most commonly, the first reaction step with organic compounds is the establishment of a peroxide intermediate [10], [38]. Then, radical species (R-O•) are produced, as the RO-OR bond is quite labile. From this point on, radical reactions propagate easily, in many cases involving 3O2 given that it readily engages in reactions with radicals. Therefore, even when the first reaction steps usually narrow down to the generation of peroxides, secondary reactions rapidly widen the available reacting species. Among these one can find organic radicals (-R•), organic alkoxyl radicals (R-O•), organic peroxyl radicals (R-O-O•), organic hydroperoxides (R-O-OH), superoxide (•O2-), hydrogen peroxide (H2O2), hydroxyl radical (•O2-), peroxynitrite (ONOO-), aldehydes, etc., to name the most important [2], [3], [4], [5], [60]. It can be concluded that 1O2 is very reactive to compounds showing double bonds, on which the oxygenation can readily proceed. And double bonds are quite abundant in organic compounds and biomolecules.
It is not my intention to review 1O2 chemistry (this topic has been under study for many decades with some important contributions cited here), but just to provide a glimpse of what can happen in a biological system, like a cell, when 1O2 is produced inside it or nearby. But, before briefly describing some of the most important chemical consequences of 1O2 production in living systems, I would like to call the reader´s attention on a phenomenon that most probably has a large impact in the biological action of 1O2: ROS interconversion. By this process one particular class of ROS reacts chemically and transforms into other ROS, which themselves can further transform into other ROS [2], [17], [49], [60]. For example, the superoxide radical (•O2-) readily oxidizes organic compounds and gets itself reduced to H2O2, which further reacts to produce hydroxyl radicals (•OH), hypochlorite (OCl-) or nitrogen dioxide (•NO2). Many other reactions are possible. This testifies on the large chemical variability a researcher must face when dealing with redox biology. In fact, it is this chemical interconversion which allows part of the flexibility necessary for efficient ROS-mediated signaling [2], [3], [4].
In regards to 1O2 and ROS interconversion, it is known that 1O2 can produce H2O2 when it oxidizes certain biomolecules like ascorbate [61]. This is a critical step to explain physiological signaling mediated by 1O2, as H2O2 is currently recognized as the main physiological signaling ROS [2], [3], [4], [5], [18], [19], [20]. A conversion from 1O2 to H2O2 makes it more accessible to canonical redox receptors (that have evolved to recognize H2O2 as a signal molecule) [18], and increases the sphere of biological activity of the former 1O2, as H2O2 has a much larger diffusion range [48]. Superoxide can be obtained directly from 1O2 by one-electron reduction [62]. Peters and Rodgers reported the single-electron reaction between 1O2 and different variants of NADH compounds [63]. Therefore, 1O2 can engage in one-electron oxidation with the main cell reducing equivalent to convert into other ROS forms. A conversion from 1O2 to superoxide was also reported due to thermal endoperoxide activation and subsequent 1O2 reaction with N,N-dimethylaniline [64]. These are proof that 1O2 can be efficiently converted to other ROS, currently accepted as signaling molecules in cell biology [3], [5], [18], [20]. Closing the circle, superoxide can promote the production of 1O2 under the right conditions, like reacting with glutathione (GSH), the principal redox potential managing compound in cells [65]. This ROS interconversion is most likely a very important parameter to take into account when 1O2 is generated directly by optical means. I will elaborate further on this in relation to redox biology studies at the end discussion.
3.2. Redox cell stress in relation to 1O2
Following the same general historical trend of research on other ROS, the study of the biological action of 1O2 dealt first with its deleterious and damaging effects on biomolecules and cells. Given its strong oxidizing nature, the production of 1O2 in living systems, even in low amounts, leads to biomolecule oxidation with concomitant alteration of functional moieties/groups and loss of cellular functions [66], [67], [68]. Thus, 1O2 can be considered a source of redox stress for the cell. One of the most important applications of 1O2, the clinical modality known as PDT, takes advantage of the differential redox stress produced by a photodynamic treatment between tumoral and normal tissue [69]. But 1O2 can be a health problem in certain circumstances, like atmospheric pollution scenarios, where sun-driven photochemistry can lead to accumulation of this and other reactive chemicals (e.g. O3 or NOx).
Cell redox stress related to 1O2 exposure has been implicated in mitotic arrest [70], cell senescence [71], inflammatory response [13], [15], [72], genetic damage [73], [74], [75], autophagic death [68], [76], apoptosis [13], [15], [68], and necrosis [15], [68]. In consequence, the study of biological responses to damaging concentrations of 1O2 is a vigorous research area, both to protect normal cells from its effect, and to optimize PDT outcomes. Also the determination of when the damage threshold for 1O2 is reached in a given system is an important question, from a basic science and health care point of view.
As mentioned before, living systems abound in biomolecules displaying double bonds and, in general, having a reductive character. The deleterious biological consequences just mentioned are a consequence of the disturbance of this reductive environment by 1O2 and other ROS. In the first place, 1O2 has been reported to oxidize a number of small biomolecules, cofactors and intermediate metabolites [61], [63], [65], [68], [77]. These oxidative events disrupt the signaling networks on which cell processes sit upon. For example, the increase in intracellular oxidation events leads to a change in the GSH:GSSG (glutathione disulfide) ratio, towards a less negative redox potential (i.e. to a more oxidized baseline) [20]. This, by itself, can start a stress response in the cell due to the redox signaling network based on the GSH:GSSG equilibrium [78].
Oxidative modifications of proteins by 1O2 are one of the most studied areas in the biological action of 1O2. The modifications with the highest biological impact are those affecting aminoacid side groups. This is because side groups provide the enzymatic capabilities of a given protein. The side groups most easily oxidized by 1O2 are: cysteine, methionine, phenylalanine, tyrosine, histidine and tryptophan [16], [77]. Thus, sulfur-containing or aromatic aminoacids have the largest reactivity towards 1O2. The consequences of oxidative damage to proteins are diverse, but always produce a lesser or greater kind of functional disruption and loss of biological action. These can range from reversible oxidation of enzymatic or regulatory sites, to irreversible chemical changes or even peptide/protein denaturation, which can compromise essential cellular structures and processes, such as the plasmatic membrane, organelles, intracellular transit, vesicles, cell movement, mitosis, etc [66], [77], [79]. Additionally, oxidized proteins can act as secondary sources of ROS and increase the damaging action of these species [71], [79].
Nucleic acids have been, along with proteins, the most studied area of biological impact of 1O2. This can easily be understood as DNA is the reservoir of genetic information of the cell, and any chemical modification of it, such as those brought about by 1O2, can lead to mutations or inadequate expression patterns. Among the elements making up DNA guanine is by far the most susceptible to oxidative damage by 1O2 [74], [80]. The product of guanine oxidation is the modified base 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG). This base modification can induce mutations and, as recently reported, also epigenetic changes, if not properly excised and repaired. The mutations are mainly a consequence of G:C → T:A transversions (although other mutations are observed in minor amounts) [81]. Additionally, 8-oxo-dG can affect histone methylation patterns [82]. So, 1O2 genetic impact goes further than direct mutation induction, and seems to have also an epigenetic deregulation role.
On a less subtle way, 1O2 has been pointed as a direct causal agent of cytogenetic alterations [70], [75]. In this case oxidative damage, both to nucleic acids and proteins, is at the root of the observed chromatin damage. On the one hand, oxidative damage to DNA is detected which signals for a rescue response. On the other hand, damage to proteins can delay this stress response, initiate its own stress response and make oxidized proteins to interact with nucleic acids in an incorrect way. This 1O2-mediated cytogenetic damage is observed, not only in the cell nucleus, but also in the mitochondria, where it has been proposed as one of the main causes of age-related oxidative modifications in this organelle [73].
Currently, oxidative damage to lipids due to 1O2 exposure is a very active research area. Biological lipids commonly display one or more double bonds, which make them idoneous chemical targets for 1O2 oxidative action [83]. After a first reaction involving the establishment of an endoperoxide, the labile intermediate rearranges to produce lipid hydroperoxides, aldehydes and radicals [84]. These reaction products display higher hydrophilicity that the original lipids, which change the membrane properties and can critically compromise its barrier function. In addition, partially oxidized lipidic compounds can participate in further rounds of oxidative chemical reactions, with the participation of ground state 3O2 [85]. These chain reaction-like oxidative waves may induce severe damage in biological membranes, compromising cell viability, and generate some amphiphilic oxidation products (e.g. aldehydes like 4-hydroxynonenal or malondialdehyde) [53], [86], which can induce damage in other cellular structures (nucleus [87] or mitochondria [88], for example), and even adversely affect nearby cells (so-called bystander effect) [89]. Cholesterol and related compounds do react with 1O2, and can start long-range damage/signaling depending on the circumstances [83], [84], [90].
3.3. Redox cell signaling induced by 1O2
In recent years, interest in the plausible signaling role of ROS as physiological compounds has emerged as a new paradigm in biology: redox biology. 1O2 is studied as one of the ROS involved in this cellular signaling. The study of the signaling roles of 1O2 goes back at least 20 years, when its involvement in stress responses was acknowledged [58], [91], [92], [93]. The oxidative action of 1O2 exposure, if not too strong, would lead to activation of stress/rescue response in cells and tissues, mostly mediated by the stress-related JNK and p38 mitogen-activated protein kinases (MAPKs), as well as NF-κB and AP-1/AP-2 [16], [72], [94], [95]. The final outcome depends on many parameters, but can range from cell adaptation, phenotypic changes, inflammatory response, senescence, autophagy or apoptosis.
However, as more studies are done on this topic, it is becoming clear that some ROS are able to subtly interfere with signaling networks in a positive way, i.e. to induce or enhance such processes as cell migration, cell proliferation, tissue regeneration, etc [5]. To do so, ROS must be generated in low doses, at particular cell localizations and for short intervals [18]. A priori, there is no definitive reason why 1O2, or its secondary ROS products, should not behave in a similar, stimulating way. In fact, several recent reports point in this precise direction [21], [33], [72], [83], [96]. The mechanisms underlying this stimulating effect are far from clear. But lessons have been learnt from other ROS, and it seems that critical cysteine residues are one of the target sensors/effectors to explain the observed results. Cell phosphatases and Src-kinases are two examples of redox regulation of cysteines. Phosphatase active sites usually have a cysteine with a catalytic role in the phosphate elimination. By oxidizing this cysteine, the catalytic activity is disrupted. A subtle oxidation (to a sulfenic state) is reversible through GSH-related activity. But stronger oxidation (sulfinic or sulfonic states) is irreversible, permanently deactivating the phosphatase. For Src-kinases the cysteines are not essential for kinase activity, but their subtle oxidation increases the enzyme activity by inducing a structural change driven by a disulfide bridge. It is known that ROS, and 1O2, are able to oxidize cysteines in phosphatases [2], [3], [16], [67], [77] and Src-kinases [41], [97]. So, these mechanisms can explain, at least initially, some of the stimulating effects of 1O2.
In any case, researchers are just starting to tread this road, and further experimental work will be necessary in the upcoming years. It is in this area that direct optical excitation of oxygen can provide very interesting results, as will be discussed in the last section of this review. So, after these introductory sections on relevant chemical and biological aspects of 1O2, we now proceed to present the photonic mechanism and available wavelengths at which 3O2 can be directly pumped to 1O2.
4. Direct optical excitation of 1O2
It is feasible to directly excite ground state oxygen 3O2 to 1O2 through absorption of light energy or photons of certain energy. Although there are several procedures to excite ground state 3O2 to the excited state 1O2, (see Section 2.2 above), direct photonic excitation is simpler: it only requires the adequate light source. In this section, I will introduce the physical mechanism behind the photonic excitation of 1O2, and the different wavelengths at which such a transition is possible. Advantages and drawbacks of each wavelength range will be discussed to offer the reader arguments to choose the best suited wavelength, depending on his or her study subject.
4.1. Optical excitation of 1O2
From the beginning it must be stressed that 3O2 does not strongly absorb light. At least, not in the wavelength range useful for research in redox biology. This might sound as a rotund spoiler of whatever will come next. However, the fact that oxygen does not absorb strongly does not mean that it does not absorb at all. It does, and its low absorption can be compensated by other means (light intensity, exposure time, “friendly-environment”, etc.). Then, why does 3O2 absorb light so weakly, and what wavelengths can we expect it to absorb?
First, molecular oxygen is a highly symmetric molecule. It is composed of two oxygen atoms which have the same electronegativity. In consequence, the molecule has no permanent dipole and for this reason 3O2 does not absorb in the infrared (IR) [98]. Second, due to its exotic outer electrons arrangement (see Section 2.1), several quantum mechanical rules forbid electronic transitions between the ground state (3O2) and the two first excited states (1O2 and 1O2 (b)) (see ref [8], [9], [10]. for theoretical arguments supporting these statements). Fortunately, this forbiddenness must be understood as a very low probability of such an event happening. Even more fortunate, oxygen interactions with other atoms or molecules partly relieve the molecular symmetry and change the electronic molecular orbitals, lifting to some extent the forbiddenness of these transitions. Experimental results show that 3O2 can be excited efficiently enough as to observe many different responses in biological systems.
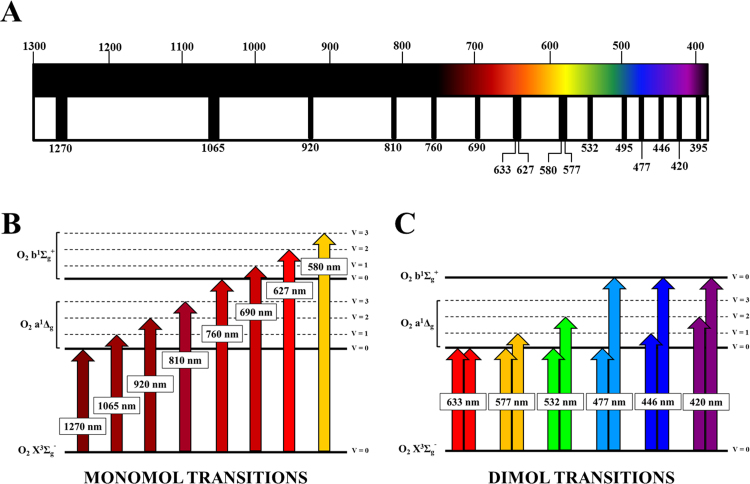
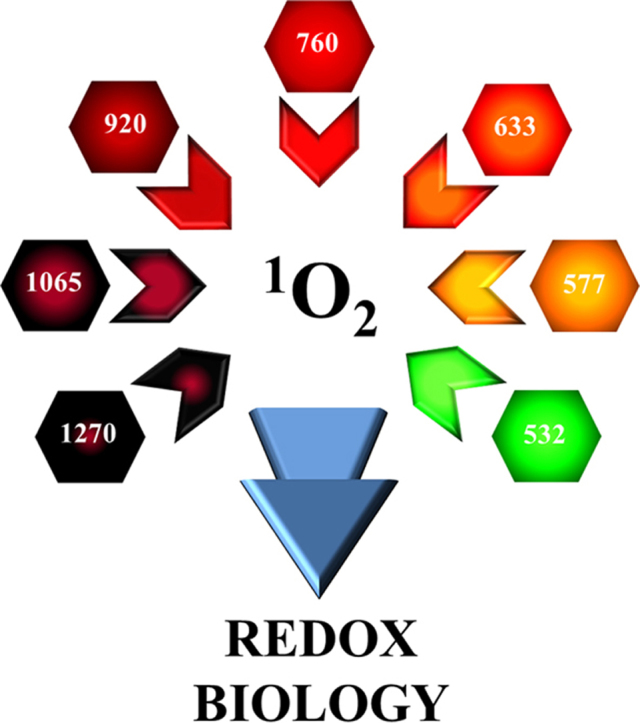
Ground state 3O2 displays several absorption bands in the IR and the visible that can produce 1O2 and 1O2 (b) (Fig. 4) [9], [22], [99], [100], [101], [102], [103], [104]. The particular absorption wavelengths that connect the different electronic-vibrational (vibronic) molecular levels are shown in Fig. 4B-C. Photon absorption in one of these bands leads to excitation of the corresponding singlet oxygen state, either 1O2 or 1O2 (b). The initial excited state can be vibrationally excited in addition (e.g. absorption at ~1065 nm produces 1O2 in the first excited vibrational level or 1O2 v = 1). In condensed media, like biological systems, this vibrational energy is quickly (~ps) dumped into the environment, and is of no further concern. It is worth reminding here that, whenever any optical transition favors 1O2 (b) generation, this species is converted to 1O2 very fast (Section 2.1), without having time to react. The absorption bands displayed in Fig. 4B are known as “monomol transitions” [9], [103]. This is because the absorption of a single photon produces a single oxygen molecule in an excited state. Thus, there is a one-to-one relationship, and one mole of photons (often designed as an Einstein) will produce one mole of 1O2 molecules. This nomenclature is not the most intuitive, but it is the established one, and researchers will find it over and over again in the pertaining literature.
Fig. 4.
Oxygen absorption bands and electronic transitions in the optical range (visible-infrared). (A) The O2 absorption bands (black stripes) between 1300 nm and 390 nm are displayed in the spectral range covering the near-infrared and visible. The number under each transition indicates the peak wavelength, and the stripe width roughly represents the bandwidth for each transition. Both monomol and dimol transitions are plotted. All numbers refer to nanometers. (B) Monomol O2 transitions represented in a Jablonski diagram. The peak wavelength for each transition is indicated. The arrows connect the initial level (fundamental) and the final levels, along with vibrational excitation (V>0) in case this occurs. (C) Dimol O2 transitions (Jablonski diagram), where two 1O2 molecules are produced (double arrows) after single photon absorption. The peak wavelength for each transition is indicated. Some dimol transitions shown in (A) have not been represented here. Data based on [35], [99], [100], [101], [102], [103], [104], [107].
A fascinating property of oxygen is that two transiently interacting ground state molecules [3O2–3O2] can absorb a single photon, and simultaneously reach excited electronic levels if enough energy is available [9], [104]. Each molecule perturbs the other electronic orbitals when in close distance, which enhances transition probabilities. Following the nomenclature introduced in the previous paragraph, these transitions are known as “dimol transitions”, as two excited molecules result after absorption of just one photon. Some observed dimol transitions are shown in Fig. 4C. In this review, I am exclusively focusing on photon absorption by molecular oxygen. But it is necessary to remark that there is reciprocity between some absorption bands and the corresponding emission bands. In other words, there is an emission band centered ~1270 nm that corresponds to the radiative deactivation of 1O2 v = 0 to 3O2 v = 0. Or emission at ~760 nm corresponding to 1O2 (b) v = 0 to 3O2 v = 0. Some practical comments concerning these emissions will be presented in Section 5.4.
In the next subsections, I will introduce monomol transitions and then dimol transitions. As a quick reference for the reader, the wavelengths, corresponding photon energies, type (monomol or dimol), and electronic states involved in each transition are summarized in Table 1.
Table 1.
Molecular oxygen optical transitions between the ground state (O2 X3Σg-) and the first two electronic excited states (O2 a1Δg) and (O2 b1Σg+). The transitions are listed in order of increasing energy (eV) (decreasing wavelength in nm). Both monomol (M) and dimol (D) transitions are shown. Data based on [35], [99], [100], [101], [102], [103], [104], [107].
| Wavelength | Photon energy | Type | Electronic transition |
|---|---|---|---|
| (M-Monomol; | |||
| D-Dimol) | |||
| 1270 nm | 0.97 eV | M | O2 X3Σg- → O2 a1Δg v = 0 |
| 1065 nm | 1.16 eV | M | O2 X3Σg- → O2 a1Δg v = 1 |
| 920 nm | 1.35 eV | M | O2 X3Σg- → O2 a1Δg v = 2 |
| 810 nm | 1.53 eV | M | O2 X3Σg- → O2 a1Δg v = 3 |
| 760 nm | 1.63 eV | M | O2 X3Σg- → O2 b1Σg+ v = 0 |
| 690 nm | 1.80 eV | M | O2 X3Σg- → O2 b1Σg+ v = 1 |
| 633 nm | 1.96 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 0 - O2 a1Δg v = 0] |
| 627 nma | 1.98 eV | M | O2 X3Σg- → O2 b1Σg+ v = 2 |
| 580 nma | 2.14 eV | M | O2 X3Σg- → O2 b1Σg+ v = 3 |
| 577 nm | 2.15 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 1 - O2 a1Δg v = 0] |
| 532 nma | 2.33 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 2 - O2 a1Δg v = 0] |
| 495 nma | 2.50 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 3 - O2 a1Δg v = 0] |
| 477 nma | 2.60 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 0 - O2 b1Σg+ v = 0] |
| 446 nma | 2.78 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 1 - O2 b1Σg+ v = 0] |
| 420 nma | 2.95 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 2 - O2 b1Σg+ v = 0] |
| 395 nma | 3.14 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 a1Δg v = 3 - O2 b1Σg+ v = 0] |
| 380 nma | 3.26 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 b1Σg+ v = 0 - O2 b1Σg+ v = 0] |
| 360 nma | 3.44 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 b1Σg+ v = 1 - O2 b1Σg+ v = 0] |
| 344 nma | 3.60 eV | D | [O2 X3Σg- - O2 X3Σg-] → [O2 b1Σg+ v = 2 - O2 b1Σg+ v = 0] |
Transitions included for completeness but not discussed in depth in the text.
4.2. Monomol transitions
Monomol transitions (Fig. 4B) represent the simplest excitation mechanism: a single 3O2 molecule absorbs one photon and gets electronically excited, either to 1O2 or 1O2 (b) states. If it is the case that 1O2 (b) is the generated species, it undergoes very fast conversion to 1O2 (as mentioned in Section 2 [37]), which is the biologically relevant molecule. Monomol absorption bands are very weak: the corresponding molar absorption coefficients are ~10−3 – 10−4 M−1 cm−1 [45], [46], [105], [106], [107] or even lower. These absolute values are 107 – 109 smaller than typical absorption coefficients for PDT PSs (but notice that PSs are commonly employed at 10−5 – 10−6 M final concentration). However, it is perfectly possible, as experiments show, to compensate the smaller absorption with a higher light flux and/or longer irradiation time.
The principal monomol bands are at 1270 nm, 1065 nm, 920 nm, 760 nm, 690 nm, and 627 nm. These bands will now be introduced in order of increasing photon energy (i.e. decreasing wavelength). Relevant biological reports employing these monomol transitions, along with experimental conditions and references, are listed in Table 2 as a quick guide for the interested reader.
Table 2.
Relevant examples of biological experiments with monomol O2 transitions. Several experimental aspects are detailed in each entry (see footnotes for more information). Each set of conditions has been obtained from a publication, with the particular reference provided in each case.
| Transition | Biological model | Light source | Wavelength | Power | Spectral control | 1O2scavenging | 1O2enhancement | Thermal control | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1270 nm | Human erythrocytes | Diode laser (cw) | 1264 nm | 0.5–2 mW | Yes | pN2 | No | No | [108] |
| T36 carcinoma in mice | Diode laser (cw) | 1264 nm | 4–4.5 mW | No | No | No | No | [108] | |
| Human skin tumors | Raman fiber laser (cw) | 1262 nm | 1.5–5 W | No | No | No | No | [23] | |
| MCF-7 breast cancer line | Raman fiber laser (cw) | 1270 nm | 1.5–5 W | Yes | -BSA | pO2 | Yes | [24] | |
| -NaN3 | |||||||||
| -pN2 | |||||||||
| CC-5 carcinoma in mice | Raman fiber laser (cw) | 1265 nm | 4 W | No | No | No | No | [26] | |
| -HaCaT | Diode laser (cw) | 1268 nm | 500 mW | Yes | α-TOC | No | Yes | [27] | |
| -Primary Keratinocytes | |||||||||
| -HeLa | |||||||||
| MCF-7 breast cancer line | Raman fiber laser (cw) | 1270 nm | 300 mW | Yes | -BSA | pO2 | Yes | [116] | |
| -NaN3 | |||||||||
| -pN2 | |||||||||
| -HaCaT | Diode laser (cw) | 1268 nm | – | No | No | No | No | [119] | |
| -Primary Keratinocytes | |||||||||
| -HeLa | |||||||||
| -HCT-116 colorectal line | Raman fiber laser (cw) | 1265 nm | 4 W | No | No | No | Yes | [28] | |
| -CHO-K ovarian line | |||||||||
| 1065 nm | NC-37 lymphoblast line | Nd: YAG laser (cw) | 1064 nm | 60–240 mW | Yes | No | No | Yes | [138] |
| E. coli bacteria | Nd: YAG laser (cw) | 1064 nm | 3–30 mW | No | No | No | Yes | [139] | |
| Human skin fibroblasts | Nd: YAG laser (cw) | 1064 nm | 1 W | Yes | No | No | Yes | [143] | |
| Double strand DNA | Nd: YAG laser (cw) | 1064 nm | 30–350 mW | No | -O2 depletion | No | Yes | [30] | |
| -Ascorbate | |||||||||
| -Lipoic acid | |||||||||
| -NaN3 | |||||||||
| S. cerevisiae yeast | Yb fiber laser (cw) | 1070 nm | 0.7–2.6 mW | No | No | No | Yes | [31] | |
| 920 nm | E. coli bacteria | Ti: sapphire laser (cw) | 900 nm | 5–19.1 mW | Yes | No | No | Yes | [145] |
| -A549 adenocarcinoma | LED (cw) | 901±69 nm | – | Yes | No | No | Yes | [146] | |
| -PtK2 renal line | |||||||||
| -U2OS osteosarcoma | |||||||||
| 810 nm | NC-37 lymphoblast line | Ti: sapphire laser (cw) | 800 nm | 60–120 mW | Yes | No | No | Yes | [138] |
| C. elegans nematode | Ti: sapphire laser (cw) | 810 nm | 240–480 mW | Yes | No | No | Yes | [147] | |
| Rat lymphoblasts | Diode laser (cw) | 808 nm | 30–100 mW | No | No | No | No | [149] | |
| Mouse primary neurons | Diode laser (cw) | 810 nm | – | No | No | No | No | [154] | |
| C2C12 myoblast line | Diode laser (cw) | 808 nm | 100 mW | No | No | No | No | [151] | |
| Rat muscle (hind leg) | Diode laser (cw) | 808 nm | 100 mW | No | No | No | No | [152] | |
| 760 nm | PtK2 renal line | Ti: sapphire laser (cw) | 760 nm | 130 mW | Yes | No | No | Yes | [161] |
| -Human sperm cells | Ti: sapphire laser (cw) | 760 nm | 70–88 mW | Yes | No | No | No | [162] | |
| -CHO line | |||||||||
| -Human sperm cells | Ti: sapphire laser (cw) | 760 nm | 70–88 mW | Yes | No | No | No | [163] | |
| -CHO line | |||||||||
| CHO line | Ti: sapphire laser (cw) | 760 nm | 88–176 mW | Yes | No | No | Yes | [136] | |
| CHO line | Ti: sapphire laser (cw) | 760 nm | 88–176 mW | Yes | No | No | Yes | [164] | |
| Human erythrocytes | Ti: sapphire laser (pulsed) | 762 nm | 200 mW | Yes | No | No | Yes | [108] | |
| Lewis carcinoma in mice | Alexandrite laser (pulsed) | 762 nm | 200 mW | No | No | No | No | [108] | |
| NC-37 lymphoblast line | Ti: sapphire laser (cw) | 800 nm | 60–120 mW | Yes | No | No | Yes | [138] | |
| C. elegans nematode | Ti: sapphire laser (cw) | 760 nm | 240–480 mW | Yes | No | No | Yes | [147] | |
| HeLa line | Ti: sapphire laser (pulsed) | 765 nm | 700 mW (pre-stage) | Yes | -NaN3 | D2O | Yes | [32] | |
| -α-TOC | |||||||||
| HeLa line | Ti: sapphire laser (pulsed) | 765 nm | 4.5–91.5 mW (on-stage) | Yes | No | No | Yes | [33] | |
| 690 nm | Human fibroblasts | Diode laser (cw) | 692 nm | 30 mW | Yes | No | No | No | [168] |
Spectral control means that the biologically-relevant wavelength has been compared to other close wavelengths in the experiments. 1O2 scavenging informs if any of the following measures have been employed to scavenge or quench 1O2 activity: BSA (added Bovine Serum Albumin); pN2 (increasing N2 pressure in the system to displace O2); NaN3 (added sodium azide); α-TOC (added α-tocopherol); O2 depletion (O2 consumption by added enzymatic oxidases). 1O2enhancement alludes to strategies to increase 1O2 activity: pO2 (pumping O2 into the system to increase its partial pressure); D2O (added deuterium oxide). Thermal control means that either the researchers have taken active measures to control the setup temperature during irradiation or, at least, they accounted for a possible photothermal activity of the actinic light in the article.
4.2.1. 1270 nm band
This transition has an energy gap of 0.97 eV (Fig. 4B). It pumps a ground state oxygen molecule into the first excited singlet state, with no additional vibrational energy (3O2 v = 0 → 1O2 v = 0). The band is relatively broad (±20 nm), although the final width depends on the particular solvent in which O2 is dissolved [105], [106], [107]. However, given the very low absorption coefficient and the band narrowness, it is advisable to try to use light sources with emission as close to the band peak as possible (see Section 5.3). This is even more advisable for all the O2 bands to be subsequently discussed. The excitation of 1O2 using 1270 nm was already introduced in the late 1960s and early 1970s, to obtain 1O2 in gas-phase oxygen at very high pressures (tens or hundreds of bars) or in different organic solvents for physical chemical studies [36], [99], [100]. These conditions were selected to make up for the low absorption at this wavelength. In these experiments oxygenation of chemical traps was demonstrated by directly exciting 1O2 optically. However, light sources emitting around 1270 nm were scarce, mainly due to lack of practical applications for this wavelength range. In consequence, experiments employing light around 1065 nm were much more common because of the coincidence of this band with the light emitted by neodymium lasers (see 1065 nm band below).
It was not until the late 1980s that this band was given proper attention, this time for biological and clinical applications [108]. Former Soviet Union researchers and clinicians employed this and other bands in what has been called the “light-oxygen effect” or LOE. In their 1999 review on this topic, Zakharov and Ivanov present a series of experimental results comparing efficiency of LOE vs. classical PDT as to biological outcomes. To study biological effects at 1270 nm they employed an InGaAsP/InP semiconductor laser. They assessed the light effect on erythrocytes suspensions by changes in the refractive index and alterations of the cell membrane. Introducing N2 in the system abrogated the changes and the cell response followed the IR absorption band of 3O2. The authors also report the use of continuous wave (cw) lasers tuned at 1264 ± 9 nm to treat tumors in mice in 1993. In these experiments the animals were exposed to light powers of 8.5 mW for times of 10 min (several days).
Then, in 2003 Krasnovsky et al. reported on the photonic production of 1O2 under experimental conditions “using laser generators approximately that power which was used in biological experiments” [109]. They employed a cw diode laser (1266±4 nm P = 55 mW) and a pulsed tunable forsterite laser (tuning range 1200–1290 nm P = 30–150 mW-average) to produce 1O2 to be trapped by aromatic compounds. The interesting aspect is that the authors measured 1O2 production by light in several atmospheric pressure air-saturated solvents at room temperature, including H2O. Their results pointed indeed to 1O2 production to explain the observed chemical trap bleaching. And positive results were obtained in H2O, even when it is, probably, the most unfavorable solvent to achieve efficient 1O2 production. Following this, in a series of publications, Krasnovsky et al. further substantiated the real possibility to achieve 1O2 generation by direct optical pumping in relevant amounts for chemical or biological studies [110], [111], [112], [113], [114]. The action spectrum for 1O2 production fitted precisely with the IR absorption band peaking at 1270 nm. The efficiency of the process was higher in organic solvents than in D2O. The presence of 1O2-quenchers, like sodium azide (NaN3) or β–carotene, effectively decreased 1O2 excitation, as did purging with N2. Of special relevance in this sense is a work published in 2008 in which 1O2 was generated by a cw semiconductor laser emitting at 1269 nm with a maximum power of 700 mW in H2O-SDS micellar systems [114]. Chemical trap photooxygenation was observed under laser irradiation in air-saturated aqueous solutions (some preliminary work was done in H2O-micelles in 2006 [112]). Therefore, 1O2 photonic generation was shown to be feasible under conditions very close to those of biological relevance.
From 2010 onwards, reports on the direct optical excitation of 1O2 at 1270 nm for biomedical goals have experienced a large increase. In 2010 a Raman-shifted laser source (1262 nm and 5.5 W) has been employed to successfully treat tumoral skin lesions in human patients [23]. It should be remarked, nonetheless, that photothermal effects cannot be ruled out due to the high power of the laser (see Section 5.6). In any case, an additive or synergic effect would be welcome, if it increases the chances of a positive clinical outcome. Also in 2010 Anquez et al. started a series of publications on direct excitation of 1O2 in the 1270 nm band under different experimental parameters. In their first contribution, 1O2 was generated using a Raman fiber ring laser (1240–1289 nm 2.5 W) in ethanol and acetone air-saturated solutions [115]. The detection of 1O2 was achieved through the use of a chemical trap. The action spectrum displayed a good fitting with 3O2 absorption spectrum in the NIR.
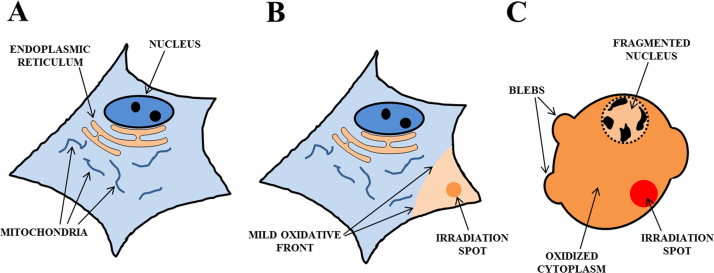
Following this first approach, Anquez et al. proved that irradiation of living human and rat tumoral cells under normal conditions (air-saturated growing medium) at ~1270 nm (100 W cm−2 1.5–3 h exposure) induced cell death [24], [25]. Cell images seem to fit with necrotic death morphology, after plasmatic membrane integrity loss and subsequent osmotic shock. Several complementary experiments point to the involvement of 1O2 as the cytotoxic agent: increase of cytotoxicity with higher pO2, decrease after N2 purging, action spectrum fitting 3O2 NIR absorption spectrum, and decrease in toxicity in the presence of 1O2-quenchers (bovine serum albumin –BSA- and NaN3). Additionally the authors took care in regards to the plausible temperature increase during NIR laser irradiation. Their results permitted to dismiss photothermal action as the damaging agent. This group has published more experimental results on 1O2 photonic excitation at 1270 nm, covering biological [116] and chemical [117], [118] models.
Other research groups have also recently explored the usefulness of the 1270 nm band. The group led by Rafailov has studied and modelled the oxidative stress induced by 1O2 excited at 1268 nm (cw semiconductor laser up to 500 mW) [27], [119], [120]. Their models support the argument that an initial 1O2 burst leads to a redox imbalance, which destabilizes cell metabolism well after actinic light exposure has ended. This is a very interesting topic, with far-reaching consequences and will be further discussed in Section 5.2. Further experiments employing a 1265 nm cw excitation have been done by Kurkov et al., where oxidative stress and regression was induced in experimental animal tumors [26], and oxidative mitochondrial and overall cell disruption was recently obtained in cell cultures [28]. As mentioned in relation with some of the experimental results presented, the main concern using this band is its photothermal potential due to IR water absorption [121]. Thermal aspects of direct optical excitation of 1O2 will be discussed together in Section 5.6 below.
4.2.2. 1065 nm band
This band has an energy gap of 1.16 eV (Fig. 4B). It involves a transition of a ground state molecule into the first excited singlet state, with concurrent excitation of its first vibrational level (3O2 v = 0 → 1O2 v = 1). The band is also relatively broad (±15 nm), again depending on the gas pressure or the particular solvent in which O2 is dissolved [103]. The absorption coefficient is smaller than the 1270 nm transition [99], [102], [103], [105]. After excitation, the vibrational energy decays very fast releasing heat to the environment, so for any practical biological purpose excitation at 1065 nm is equal to excite at 1270 nm. Two are the main advantages, one fundamental and the other one practical, of using 1065 nm in biological research: the tenfold reduction in water absorption as compared to 1270 nm [121], and the coincidence of this absorption band with the emission from commercially-available neodymium-based lasers (~1064 nm).
Experiments employing this band to excite 1O2 started in the 1970s, basically aiming to address several physical chemical parameters like chemical reactions and reaction rates [99], [100], [122], [123], [124], [125], oxygen luminescence [101], [126], [127], [128], or even oxygen lasing in the red and IR [129] (for more recent studies on molecular oxygen lasing schemes see also [130], [131], [132], [133]). In fact, one of the first determinations of 1O2 reaction rates with aminoacids was obtained by optically pumping 1O2 at 1064 nm in D2O, which thereafter reacted with histidine, tryptophan, methionine, tyrosine and alanine (in this order of decreasing reactivity) [134].
Due to their availability and reliability, Nd-based lasers have found an important niche in biological studies as light sources for optical tweezers. Optical tweezers allow the trapping and controlled movement of microscopic entities (e.g. cells) by focusing the light or making adequate interference patterns. At ~1064 nm photon absorption by biological chromophores is negligible as long as one stays in the linear excitation regime, i.e. irradiance < MW cm−2. Thus, Nd lasers, mainly Nd-YAG, are widely employed as sources for optical tweezers. However, several publications have reported chemical and biological damage at relatively low light intensities in optical traps at this wavelength [30], [31], [135], [136], [137], [138], [139], [140], [141], [142]. In many of these publications the source of damage has been ascribed to direct thermal action (due to water absorption), organic chromophore excitation in the limit of the absorption band (e.g. cell cytochromes) or biphotonic excitation. Very few articles point directly to 1O2 excitation as a plausible source of damage, although some of them show cell damage action spectra that fit reasonably well with the absorption spectrum of 3O2 and invoke ROS as a general disruption mechanism [136], [137], [138], [142]. On the other hand, experiments by Landry et al. provide direct evidence for 1O2 production in an optical tweezers setup [30]. The authors show that depleting the medium of O2 eliminates the oxidative damage to a DNA molecule stretched between two microscopic beads trapped by the optical tweezers. Oxidative damage to DNA is linearly proportional to laser power (i.e. one-photon process) and addition of 1O2 quenchers (e.g. NaN3 and ascorbic acid) greatly diminishes it too. However, the authors point to the polystyrene beads employed to tether the DNA as the source of 1O2. Although not completely negating this possibility, the alternative view that 1O2 is being directly excited by 1064 nm photons should be considered. As other IR wavelengths are employed in optical tweezers, and some of them overlap 3O2 absorption bands, I will discuss further the oxidative damage in optical traps in Section 5.5.
Some research groups have focused on this wavelength range to directly perturb living systems. Abrahamse et al. have compared 1064 nm vs. 632.8 nm and 830 nm exposure of fibroblast cultures [29], [143], [144]. Some cell damage was observed a few days after laser exposure especially in the 1064 nm group. At high light doses the two groups displaying higher levels of damage were 632.8 nm and 1064 nm, a dimol (see below) and monomol O2 absorption bands. The authors theorize that this damage is not of thermal origin and hint that ROS could be the source. Although this group has later focused in the biological effects of shorter wavelengths, their former results with 1064 nm light indicate that there is a significant biological activity at that wavelength. Thermal issues can be something to consider, but water absorption is one order of magnitude lower at 1065 nm in respect to 1270 nm. Therefore 1065 nm seems a priori a better excitation source for redox biology studies concerning 1O2.
4.2.3. 920 nm band
This band corresponds to the transition (3O2 v = 0 → 1O2 v = 2) with a photon energy of 1.35 eV. The transition probability decreases exponentially the higher the final vibrational level and, in consequence, this wavelength has been scarcely employed for biological studies. It displays a low absorption by water but nevertheless only half that of 1065 nm light [121], which does not compensate for the decrease in the 3O2 absorption coefficient. Nowadays is easier to find light sources at ~920 nm but sources at ~1065 nm are still much more widespread.
This wavelength range is employed in optical traps where some biological side effects are reported. Liang et al. studied the action spectrum for cell perturbation in the range 700–1100 nm [136]. They found a marked decreased in cell clonal growth at ~900 nm (along 1064 nm and 740–760 nm). The authors dismiss a thermal mechanism of damage and suggest that biphotonic processes could explain the observed biological effect. Two articles studied the optimal NIR region for bacterial (E. coli) optical trapping. Neuman et al. observed a clear deleterious effect on bacterial movement (taken as a proxy of viability) around 915 nm [137]. The effect was dependent on oxygen presence and showed one-photon dependence. The authors directly point to 1O2 as a most plausible source of damage although photosensitized by some “sensitizer molecule”. They acknowledge that 1O2 can be directly photoexcited but, most surprisingly, they state that “…the absorption spectrum for molecular oxygen does not resemble the action spectrum for E. coli.”. A further paper was published in 2008 assessing again the action spectrum for bacterial photodamage between 840 and 930 nm [145]. There is a marked increase in bacterial metabolic inactivation and killing at 900 and 930 nm (more severe at this wavelength) when compared to 840 and 870 nm. The photodamage is linear with light intensity although a hypothesis involving biphotonic excitation of GFP (which was employed to label the bacteria) was advanced to try to explain the lack of putative chromophore. Oxygen is not considered and, in the end, the authors state: “A specific photochemical cause of photodamage eludes us—the spectrum is not consistent with water or E. coli or the media.”. Additional comments of 1O2 generation in optical traps will be provided in Section 5.5.
Recently Spitler and Berns have published interesting results on cell photostimulation and in vitro wound healing [146]. They employed light emitting diode (LED) arrays to increase cell proliferation and migration in several cell lines. The most successful excitation was obtained with LEDs emitting at 901 nm (peak), but with a very broad emission spectrum (full bandwidth at half maximum –FWHM- of 69 nm), fully overlapping the 920 nm oxygen band. Generation of 1O2 or other ROS is not mentioned, but light absorption by elements of the mitochondrial electron transport chain (cytochrome c oxidase) is proposed as a possible chromophore to explain the results (see Section 5.3 for more on O2 absorption vs. endogenous chromophores). Anyway, this article highlights the use of LEDs as alternative light sources to lasers for O2 optical excitation (Section 5.3).
4.2.4. 810 nm band
This band corresponds to the transition (3O2 v = 0 → 1O2 v = 3) with a photon energy of 1.53 eV. It has been widely employed in photobiological studies given the availability of diode lasers emitting at 808 nm. In addition, wavelengths between 800 and 850 nm show very low absorption in biological systems, due to the lack of endogenous chromophores absorbing in this range and the still very low water absorption in this region of the NIR (see for more on the so-called biological window). At this wavelength the transition probability is extremely small and, in fact, absorption by other chromophores, like cytochromes, should complementary be considered to explain the biological responses observed.
This wavelength range is popular in optical traps due to the very low level of damage observed in bacteria [137], eukaryotic cells [136], [138] and in whole organisms (C. elegans) [147]. Nevertheless, the light is not innocuous. Under the right irradiation conditions cell growth inhibition has been reported. Under long irradiation times the growth and proliferation of a human glioblastoma cell line was significantly inhibited [148]. Studies led by Fonseca have found evidence of genetic damage [149], increased mRNA and protein expression involved in DNA repair and genomic stabilization [150], and increasing occurrence of both apoptosis and necrosis [151] in white blood cells and myoblasts exposed to 808 nm light. Both gross chromatin alterations (comet assay) as well as base-level oxidative modifications (8-oxo-dG) were observed. These genetic alterations, although not exclusive of 1O2 action, strongly point to its production during light exposure. Quite recently this group has found concerning evidence of ploidy alterations (changes in the cell chromosomal set) and a trend in DNA fragmentation (not statistically significant) in muscles exposed to 808 nm light [152].
In contrast, Yu et al. have found a protective role of 810 nm light in an oxygen-glucose deprivation neuronal model in vitro [153], [154]. However the light fluence employed (3 J cm−2) with a cw diode 810 nm laser is between 3 and 23 times smaller than that employed by Fonseca et al. Again, this pinpoints the importance of dose in inducing stimulating or deleterious biological effects.
4.2.5. 760 nm band
This band presents the peculiarity that the produced singlet oxygen species is the second excited state (3O2 v = 0 → 1O2 (b) v = 0) [35], [36], [37]. The transition energy is 1.63 eV and the bandwidth is fairly narrow (±10 nm) [9], [46], [113]. In aqueous solutions its decay lifetime is very short, on the order of picoseconds, and it can be assumed that all 1O2 (b) produced is converted to 1O2 with unity efficiency [37]. The direct optical excitation of 1O2 (b) has been successfully employed both in past decades, and recently in the field of physical chemistry [6], [7], [99], [100], [102], [155], [156], [157], [158], [159], [160].
Research done mainly in the last decade has shown that it is feasible to directly excite 1O2 (b) at ~760 nm in different solvents [46], [106], [110], but particularly in H2O, D2O and aqueous solutions [32], [46], [105], [107]. In consequence, given H2O role as universal solvent in biological systems, the production and biological activity of 1O2 (b) after exposure to 760–765 nm light was proposed and demonstrated recently [32], [33], [34]. Experiments were done with a pulsed Ti:sapphire laser with different average powers and irradiation times (5–100 mW and 10 s – 10 min) focused inside HeLa cells. As a result, different cell responses have been produced, which ranged from fast necrosis to cell proliferation stimulation [33]. Additionally, irradiations were done just outside cells and necrosis was also achieved, an important issue in the study of the sphere of action of 1O2 (b). The primary role of 1O2 in the biological responses was demonstrated by an enhanced effect in solutions partly prepared with D2O, the inhibitory action of 1O2-quenchers (NaN3 and α-tocopherol), the negligible effect of catalase (H2O2 quencher), and the establishment of a biological action spectrum for cell death, in almost perfect coincidence with 3O2 absorption between 730 and 800 nm. Additional evidence was obtained when a cw Ti:sapphire laser induced necrosis in single cells at 763 nm, but not at 800 nm, thereby ruling out any non-linear optical process (Blázquez-Castro, Haro and Jaque, unpublished results).
But the biological action of light in the 750–770 nm range has been observed much earlier, although in practically all the cases it went unrecognized as the consequence of the direct optical excitation of oxygen. In the early 1990s optical trapping was rapidly developing as a biological manipulation technique. Researchers were interested in finding the safer wavelength range to study samples. So efforts to establish an action spectrum of biological damage (expressed in different ways, from loss of mobility to outright cell killing) were done (some results concerning this have already been presented in previous O2 bands). Several publications came to the conclusion that the region around 765 nm should be avoided. Deleterious biological effects observed to support this argument include positive genetic comet assay [138], heat shock response in C. elegans [147], chromosomal damage [161], loss of spermatozoa mobility [162], loss of cell cloning efficiency [136], [162], [163], propidium iodide internalization [163], and giant cell/coenocyte formation [164]. Most publications point to some biphotonic effect as the cause of these negative responses, some also mentioning the possibility that ROS (in general) could be involved. Somewhat surprisingly König et al. found no differential biological effect in cell cloning efficiency when cells were exposed to 760 nm vs. 730 and 800 nm [165]. A possible explanation for this result can be that the irradiances employed in the experiments were relatively high (1012 W cm−2) which, as pointed by the authors, can lead to plasma generation inside the cell. Damage due to plasma formation would surpass any effect 1O2 production could induce.
The recent biological results obtained at ~760 nm, both damaging [32] and stimulating [33], seem to contradict the very low 3O2 absorptivity and small 1O2 amount generation. It can happen, however, that cells are more susceptible to 1O2 than suspected earlier. I will discuss this in Section 5.2. Additionally, as timely pointed out recently by Krasnovsky and Kozlov, the absorption coefficients of 3O2 at 1273 nm and 765 nm in aqueous solutions are practically equal [107]. More arguments in favor of employing one or the other wavelength band in redox biology research will be provided in Section 5.3. Some preliminary research in tumor regression was done in the early 1990s with a pulsed alexandrite laser emitting at 762 nm [108]. The researchers observed a significant tumor growth suppression and average lifespan extension of 50% in the treated mice.
4.2.6. 690 nm band
Absorption in this band promotes the following transition (3O2 v = 0 → 1O2 (b) v = 1). The energy gap is 1.80 eV, in the far red optical spectrum. Few publications cite results dealing with this band, which mainly involve spectroscopy and 1O2 quenching studies [157], [158], [166], [167]. The transition probability is quite small, and it overlaps greatly with endogenous chromophores present in living cells. Some biological results were published by Almeida-Lopes et al. after laser diode exposure (one of them emitting at 682 nm) of human gingival fibroblasts in vitro [168]. Growth curves point to a subtle stimulating effect of 692 nm over controls and cultures exposed to 786 nm during the first 4 days. The effect disappears by day 6 post treatment. Precisely due to this band overlap with endogenous chromophores its use in redox biology studies is discouraged, as it is more difficult to reach valid conclusions on the working mechanisms, both chemical and biological.
4.3. Dimol transitions
These transitions excite two singlet oxygen molecules while just one photon (hν) is absorbed by the momentary complex formed by two ground state molecules: [3O2 - 3O2] + hν → [1O2 - 1O2] [104]. The singlet oxygens produced can be vibrationally excited or not, or one can be in the first excited state (1O2) and the other in the second excited state (1O2 (b)). The dimol transitions are listed in Table 1 and displayed in Fig. 4C. As these transitions depend on the temporal establishment of the [3O2 - 3O2] collision pair, the absorption coefficient depends on the square of the oxygen partial pressure (pO2). The pO2 in air-saturated aqueous solutions is around 0.27 mM, thus it follows that dimol bands are relatively weak in comparison to the monomol transitions introduced previously. This is an important argument discouraging their use in redox biology in favor of monomol excitations. Another argument is that dimol transitions cover a range of wavelengths between 633 nm in the visible and 344 nm in the UV (see Table 1). These wavelengths overlap the absorption bands of numerous endogenous chromophores, from cytochromes in the far visible [169], [170] to cofactors and aminoacids in the UV [171], [172]. Therefore it becomes very difficult to discern which chromophores and ROS get involved in a particular biological response after light exposure at these wavelengths. Nevertheless some of them are very popular, in particular light of 633 nm, in the so-called low-level laser therapy (LLLT), and I will introduce briefly the main dimol transitions. Relevant biological works employing dimol transitions are listed in Table 3.
Table 3.
Relevant examples of biological experiments with dimol O2 transitions. Several experimental aspects are detailed in each entry (see footnotes for more information). Each set of conditions has been obtained from a publication, with the particular reference provided in each case.
| Transition | Biological model | Light source | Wavelength | Power | Spectral control | 1O2scavenging | 1O2enhancement | Thermal control | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 633 nm | Human erythrocytes | Dye laser (pulsed) | 633 nm | – | Yes | No | No | No | [108] |
| Human erythrocytes | He-Ne laser (cw) | 632.8 nm | 38–50 mW | No | pAr | pO2 | Yes | [108] | |
| NC-37 lymphoblast line | He-Ne laser (cw) | 633 nm | 1.2 mW | No | No | No | No | [177] | |
| A2058 melanoma line | He-Ne laser (cw) | 632.8 nm | 7 mW | No | No | No | Yes | [178] | |
| Human skin fibroblasts | He-Ne laser (cw) | 632.8 nm | 18.8 mW | Yes | No | No | Yes | [143] | |
| MCF-7 breast cancer line | He-Ne laser (cw) | 632.8 nm | 14 mW | No | No | No | No | [141] | |
| -A549 | Diode laser (cw) | 635 nm | – | No | -SOD | No | No | [181] | |
| -EMT6 | -NAC | ||||||||
| -ASTC-a-1 | |||||||||
| -EMT6 tumors in mice | Diode laser (cw) | 635 nm | – | No | No | No | No | [181] | |
| -ASTC-a-1 tumors in mice | |||||||||
| -A549 adenocarcinoma | LED (cw) | 637±17 nm | – | Yes | No | No | Yes | [146] | |
| -PtK2 renal line | |||||||||
| -U2OS osteosarcoma | |||||||||
| Clinical trials in women | Diode laser (cw) | 635 nm | – | No | No | No | No | [180] | |
| 577 nm | Human erythrocytes | Dye laser (pulsed) | 585 nm | 70–300 mW | Yes | No | No | Yes | [108] |
Spectral control means that the biologically-relevant wavelength has been compared to other close wavelengths in the experiments. 1O2scavenging informs if any of the following measures have been employed to scavenge or quench 1O2 activity: pAr (increasing Argon pressure in the system to displace O2); SOD (added Superoxide Dismutase); NAC (added N-acetyl cysteine). 1O2enhancement alludes to strategies to increase 1O2 activity: pO2 (pumping O2 into the system to increase its partial pressure). Thermal control means that either the researchers have taken active measures to control the setup temperature during irradiation or, at least, they accounted for a possible photothermal activity of the actinic light in the article.
4.3.1. 633 nm band
Absorption of one 633 nm photon promotes the following dual transition: [3O2 - 3O2] + hν → [1O2 v = 0 - 1O2 v = 0]. Basically it is the dimol counterpart of the 1270 nm monomol transition (two 1270 nm photons have the same energy as one 633 nm photon), with an energy of 1.96 eV. This transition has been employed to assess physical and/or chemical parameters, mainly in high pressure oxygen although there are studies in organic solvents too [99], [101], [123], [173], [174], [175], [176].
Its popularity in biological studies comes from the fact that the band coincides with the emission of the very common Helium-Neon (He-Ne) laser at 632.8 nm. Thus, He-Ne laser availability has made studies at this wavelength quite accessible. There are literally hundreds of scientific publications on the biological action of He-Ne laser. Most of them study the photomodulation and photostimulation of cells, tissues and animals within the frame of LLLT [108], [141], [143], [177], [178], [179], [180] although some studies also report on the damaging action when high light doses are employed [29], [181]. LLLT is currently a very active and broad research and clinical area. It is therefore outside the scope of this review which tries to focus explicitly in the direct excitation of 1O2. The interested reader can consult some of the cited references for more specific sources and information on this very appealing topic (see also the review by Chung et al. for an interesting introduction to LLLT [182]).
It is worth mentioning that, by pure chance, the 633 nm dimol transition overlaps with a 627 nm monomol transition. This monomol band corresponds to the initial excitation of 1O2 (b) v = 2 [157], [158], [167]. However its absorption coefficient is very low. But it seems interesting, in regards to the mechanistic action of LLLT, to keep in mind that exciting at ~633 nm can in fact excite 1O2 by two complementary transitions: a monomol and a dimol.
4.3.2. 577 nm band
Absorption of a ~577 nm photon leads to the following transition [3O2 - 3O2] + hν → [1O2 v = 1 - 1O2 v = 0]. One of the 1O2 molecules is produced in the first vibrational state which rapidly deactivates to the v = 0 level releasing heat [174], [183]. This wavelength range is routinely employed in the photothermal treatment of vascular disorders making use of pulsed dye lasers [184]. The possibility that a partial effect is due to 1O2 excitation cannot be ruled out, but evidence points to a purely thermal mechanism of action. However there is at least one example in which a biological response has been measured and assigned to direct 1O2 excitation at ~577 nm. Armichev et al. reported on changes in the plasmatic membrane permeability of red blood cells exposed to a tunable dye laser emitting between 570 and 590 nm [185]. The permeability alterations were wavelength-dependent and followed O2 absorption. The results are partially reproduced in a review by Zakharov and Ivanov [108]. In connection with these biological results, and as it occurred to the 633 nm dimol band, the 577 nm dimol shows a fortuitous overlap with a monomol band at ~580 nm [183]. This monomol corresponds to the initial excitation of 1O2 (b) v = 3 [186], [187]. Authors of ref [108], [185]. advanced that, most probably, the combination of both monomol and dimol absorption explained the observed effect on red blood cells.
4.3.3. Other dimol bands
There are other dimol absorption bands in the visible and the near UV (see Table 1). I am just going to mention them, but not develop them further. This is because more and more compounds absorb light when irradiation wavelengths become shorter, and it becomes a formidable task to assign a single causal agent to a given biological response. In the green spectral region (~532 nm) there is a third dimol band ([3O2 - 3O2] + hν → [1O2 v = 2 - 1O2 v = 0]). Frequency-doubled Nd-based lasers emit at 532 nm and thereby are excellent sources to excite this band. A fourth dimol band exists at ~495 nm ([3O2 - 3O2] + hν → [1O2 v = 3 - 1O2 v = 0]). Another dimol absorption occurs around 477 nm ([3O2 - 3O2] + hν → [1O2 v = 0 - 1O2 (b) v = 0]). It has the particularity that both the first and second excited singlet states are simultaneously produced after photon absorption. Further dimol bands can be observed at 446, 420, 395, 380, 360, and 344 nm. They are the result of different combinations of 1O2 and 1O2 (b) being produced in different vibrational states (see Table 1). These bands overlap with a large cohort of endogenous biological chromophores and, from my point of view, offer little advantage in redox biology research due to the complexity of photochemical reactions that can take place. Nevertheless, it can be instructing to mention all these wavelengths for the interested reader.
5. Outlook
In what follows, I will briefly discuss some research and applied concepts to be taken into account when direct optical excitation of O2 is implemented in redox biology studies. Also I will include comments and suggestions in regards to some scientific areas that share methodological aspects with this technique, introducing different points of view to answer some issues in those areas. To complement this section, the absorption coefficients for 3O2 in several biologically-relevant solvents, recently published, are listed in Table 4. With this data researchers can estimate order-of-magnitude 1O2 production rates and doses, when considering and planning redox biology experiments using direct optical excitation of 1O2.
Table 4.
3O2 absorption coefficients for some wavelengths in different solvents. Data for H2O and other biologically-relevant solvents are provided with corresponding references.
| Solvent | Wavelength | Abs. coefficient (10−3 M−1 cm−1) | Reference |
|---|---|---|---|
| H2O | 1273 nm | 1.5 | [107] |
| 765 nm | 1.1 | [107] | |
| H2O + 0.2 M SDS | 1270 nm | 1.2 | [105] |
| 760 nm | 1.0 | [105] | |
| D2O | 764.3 nm | 1.34 | [46] |
| Ethanol | 1270 nm | 2.2 | [105] |
| 1273 nm | 2.8 | [107] | |
| 760 nm | 0.97 | [105] | |
| 765 nm | 1.06 | [107] | |
| Acetone | 1270 nm | 2.3 | [105] |
| 1273 nm | 3.2 | [106] | |
| 1273 nm | 2.9 | [107] | |
| 760 nm | 0.88 | [105] | |
| 765 nm | 0.88 | [106] | |
| 765 nm | 0.96 | [107] | |
| 763.6 nm | 0.88 | [46] | |
| Methanol | 764 nm | 0.71 | [46] |
5.1. Methodological aspects of direct optical 1O2 excitation
The direct excitation of 1O2 allows for robust and essential control experiments to be done. This is a strong methodological asset of this approach, and adequate comparison among experimental conditions must be implemented by all means, if one is to conclude the causal role of 1O2 in the biological experiments. These control experiments are affordable due to several reasons. First, if 1O2 is at the origin of a given biological response, then a strong wavelength-dependent effect should be observed. In other words, the response should diminish, and finally disappear, the farther the exciting light is from the peak of the absorption band. This has been observed, for example, when HeLa cells were efficiently damaged under 765 nm excitation, but no deleterious effect was observed once the exciting laser was tuned farther than 15 nm to each side of the 765 nm absorption peak [32]. Similar control-light examples in chemical and biochemical experiments have been done recently by Krasnovsky et al. [105], [106], [107], [110], [111], [112], [113], [114]. Thus, this photonic methodology allows for control-light experiments under the same experimental conditions by just changing the wavelength. In fact, redox biology experiments done under this methodological approach should always provide for a sham-irradiation control done at as-close-as-possible wavelengths to the one producing 1O2 (e.g. using light at 775 or 780 nm along the “active” 760–765 nm). Oxygen bands are very narrow in comparison to other chromophores which may overlap in absorption (including thermal effects by water excitation). Therefore, biological responses observed under light excitation outside the O2 bands should make the experimenter suspicious about the possibility that another chromophore is actually the source of the biological outcome.
A second important advantage is that 1O2 lifetime can be modulated, to a certain extent, by several chemical compounds. On one side 1O2 lifetime can be enhanced in living systems by D2O addition. The energy transfer to D2O is much less efficient than to H2O. The lifetime in pure D2O is ~68 µs [46] in comparison to the 3–4 µs in H2O. However due to chemical reactions and physical interactions it is of the order of 35 µs in the intracellular milieu [47], [48]. In any case this order of magnitude increase in lifetime affords for a noticeable enhancement of 1O2 biological impact: time for necrotic cell killing was reduced four times in D2O-based incubation medium [32]. On the other side 1O2 lifetime can be reduced by addition of physical and/or chemical quenchers, so that its biological action is diminished. The compound NaN3 is able to deactivate 1O2 without engaging into chemical reaction. The decay is accompanied by heat release. Unfortunately NaN3 is poisonous for the mitochondrial electron transport chain but cells tolerate mM final concentrations for several hours. Adequate NaN3 controls should provide for biological effects due to this compound. Cell media made of different mixing percentages of H2O:D2O can also provide for different rates of physical 1O2 quenching, therefore providing insight into 1O2 biological action. Finally several compounds react directly with 1O2 in a sacrificial way, being oxidized themselves instead of cellular components. However results with these compounds should be interpreted with care, as new ROS can form precisely due to these very chemical reactions. For example, ascorbate reacts with 1O2 leading to the production of the superoxide anion radical and H2O2 [61].
Most biological studies done so far rely on lasers to excite directly O2 (for the reasons behind this see Section 5.3 below). Due to the high coherence and directivity of lasers, it is possible to focus the light beams almost to the diffraction limit. For the wavelengths discussed in this review this means that light spots in the 0.5–1 µm diameter range are affordable. In turn this translates into a very high spatial control over the subcellular domain where 1O2 is being produced. This control opens the way to study subcellular effects driven by 1O2 by irradiating particular organelles or areas. It also allows for studies on the diffusion of 1O2 inside the cell and the differences/similarities of intracellular vs. extracellular generated 1O2 [32]. Something to consider is that the diffraction spot dimensions are commonly referred to the lateral (x and y axis) ones. The depth (z-axis) limit is larger as the beam focuses and then defocuses above and below the focusing spot. In consequence, there is a roughly conical volume above and below the focusing spot where 1O2 is directly excited too [33]. An interesting experiment partly avoiding this issue would be to make use of the total internal reflection effect at an adequate wavelength, to study 1O2 production in a very thin slice just outside/inside the cell. This can be an adequate approach to study the impact of 1O2 on the plasmatic membrane, membrane proteins and receptors, etc. Also to take into account is the fact that actinic light gets scattered and diffracted, especially in cellular environments that show refraction index changes and micrometric structures. As direct 1O2 excitation is a one-photon process this scattered light will produce some 1O2 outside the region of interest.
To avoid unwanted and interfering results due to non-linear optical effects, the use of pulsed lasers should be avoided whenever possible. Biphotonic or multiphotonic excitation because of short laser pulses (nanosecond or shorter) can lead to excitation of organic chromophores (which can photosensitize 1O2 but also other ROS as in the classical photodynamic effect) [34] or induce plasma generation that produces a number of ROS [188]. These effects obscure the role 1O2 can play in redox biology by adding additional parameters the researcher must account for.
5.2. Redox biology with optically excited 1O2
Direct excitation of 1O2 can be employed to induce cellular redox eustress (stimulation [5]) [33], [180], redox distress (stress response and damage [5]) [28], [33], [119], [120], cell death [24], [27], [32], [33], [116], and, seemingly, it can be put to clinical use, at least for some kind of tumors [108], [189]. A puzzling aspect of 1O2 action at all this levels is the apparent high biological susceptibility to small 1O2 amounts: given the very small absorption coefficient of 3O2 and its low concentration in cells and tissues, it is not easy to understand how relatively short irradiations can have such a biological impact. To answer this question is, in my opinion, one of the biggest issues to deal with in regards to 1O2 action in redox biology. A mechanism that can partly address this is the ROS-induced ROS release (RIRR) phenomenon, a term initially introduced to explain the observed chronic increase in ROS production by mitochondria well after an initial oxidative insult has been applied [190], [191]. In many of the original studies on RIRR the oxidative treatment was ionizing radiation. In RIRR certain oxidative modifications of the affected mitochondria induce leaks and a faulty behavior in the respiratory process. As a consequence, more ROS are produced by the mitochondria, which in turn deal more oxidative damage and further the inefficient respiration process. This positive feedback perpetuates the deleterious cycle which can end by killing the cell or transforming it. Photoaging processes related to RIRR are known to be driven by 1O2 [73]. Recently the process has been expanded to explain similar events not exclusively linked to mitochondrial ROS production [192]. That an initial “oxidative spark” by optically excited 1O2 can reach the threshold for significant local structural/functional modifications, that subsequently lead to an increased ROS production, seems feasible in view our current knowledge of the cell physiology and RIRR. Putative ROS sources for such an 1O2-driven RIRR can be the mitochondria [28], [193], the family of NAD(P)H Oxidases (NOX) [41], [194], the lipoxygenases [195] or, most probably, a mixture of all of them. The ROS interconversion process is surely playing a role in this 1O2 susceptibility, as it can transform a short-lived ROS like 1O2 into longer lived species like H2O2. In fact, it has been reported that the bystander effect after a photodynamic treatment is mediated by H2O2 [196] and other diffusing mediators like lipid peroxidation products [197]. The group led by Rafailov has modelled the impact of an initial 1O2 pool (generated by 1270 nm light exposure) on the subsequent ROS increased production and redox stress in normal and tumoral cells [27], [119]. Recently, it has been proposed that extracellular generation of 1O2 can, under the right circumstances, derive into even more 1O2 production through enzymatic processes and catalase inactivation at the cell membrane [194].
It is interesting to remark the potential importance of lipid oxidation products as signaling and damaging agents. Lipids are the bulk components of biological membranes. Oxygen solubility is higher in organic hydrophobic solvents so its concentration is more elevated in biological membranes. At the same time, 1O2 lifetime is longer in this kind of solvents and it is reactive towards lipidic compounds [83], [84]. Therefore, it can be expected that lipid oxidation products derived from 1O2 membrane reactions will be one of the main oxidation products to be generated during and right after exposure to one of the actinic wavelengths presented. A particular compound within these oxidation products, 4-hydroxynonenal (4-HNE), has some interesting properties that can partly explain the paradoxical low-dose vs. high-dose biological effects of 1O2. At high doses 4-HNE is cytotoxic and mutagenic, promotes lipid oxidation chain reactions and, due to its amphiphilic aldehyde character, can diffuse from membranes to other cellular locations through the aqueous compartments [86], [87], [88], [89], [198], also supporting the bystander effect. However, it has been reported that at low doses (micromolar or lower) 4-HNE has physiological signaling roles, like cell mitosis regulation and enhancement [199], [200], [201]. Deeping in the mechanisms behind this cell stimulation Ramana et al. found activation of protein kinase C, NF-kB and AP-1 after low concentration 4-HNE exposure [202]. Another two groups have found activation of the Src kinase pathway under similar circumstances [203], [204]. Mild photodynamic treatments induce cell stimulation partly through activation of Src kinase [41], [42], [43], which is a prototypical example of kinase regulation by redox modulation [97]. Intracellular levels of 4-HNE modulate GSH concentration and, in consequence, the overall reduction potential (Ered) of the cell [200], [202], [205]. Additionally, 4-HNE and other lipid oxidation products can increase ROS production by interfering with the mitochondria [88] or NOX [206]. This brings again the RIRR argument introduced previously in this section. In summary, lipid oxidation products, in particular 4-HNE, may work as efficient signal transducers for 1O2-driven redox processes in the cell.
An experimental principle to keep always in mind is that direct optical excitation of 1O2 guarantees that the only ROS produced in the first place is 1O2. As obvious as this might sound, it can help answer fundamental questions in redox biology. For example, in connection to the RIRR phenomenon just discussed or the ROS interconversion processes presented. Does redox signaling show ROS-specificity or is it supported by an unspecific action mechanism? It seems that subtle general modulation of the cellular Ered explains many redox signaling effects [19], [20], [207], [208]. As (Ered) is tightly dependent on the ratio GSH:GSSG [3], [20], [78], it is reasonable to think that different cell responses can be obtained by judiciously providing different “oxidative levels”, irrelevant of the particular ROS involved in the induction of the oxidative action, but attending to the location, amount and duration of them. This is, at the moment, highly speculative, but it has been systematically proved that different ROS (H2O2, •O2- or 1O2) induce very similar cell responses [2], [5], [18], [209].
Following this theoretical argument, a schematic of two opposite cell responses (mitosis stimulation vs. apoptosis) that can be induced by direct optical 1O2 excitation is shown in Fig. 5. The interphase rest cell state is shown in Fig. 5A. Differences exist in the Ered value among different cell domains. These have been represented as a color code: cooler colors for more negative Ered (more reduced) and warmer for less negative (more oxidized) values. The nucleus, for example, shows a reduced environment (deep blue) to preserve genetic material against oxidative modifications. When a low dose optical treatment is applied to a subcellular spot (Fig. 5B), low amounts of 1O2 are produced that locally decrease the Ered value (deep orange spot). ROS diffusing from this spot induce a mild oxidative wave (light orange) that spreads to nearby cell locations. Under the right time-dose conditions, this ROS wave can be transduced to a mitotic signal [21], [33], [41], [210]. If the irradiation is continued and/or the light dose is high enough (Fig. 5C), the amount of ROS in the illuminated spot becomes large (red spot) and a generalized oxidative state imposes in the intracellular milieu (deep orange cytoplasm and light orange nucleus). In this particular case, the irradiated cell is shown undergoing apoptosis. Optical excitation of 1O2 offers the additional possibility to study the alternative biological outcomes of irradiating different intracellular domains and structures/organelles. Interesting results in this area have already been reported using a more classical photodynamic approach irradiating different cytoplasmic areas [211], and ionizing radiation at mitochondria [190].
Fig. 5.
Model for 1O2 biological modulation depending on the actinic light dose. (A) Under physiological conditions, the cell redox potential is different among cellular organelles. The nucleus and mitochondria show strongly reducing environments (deep blue). The cytoplasm has moderately reducing conditions (light blue), and the endoplasmic reticulum is mildly oxidizing (light orange). (B) When low amounts of 1O2 (deep orange) are produced by a subcellular optical beam (irradiation spot), a transient mild oxidative wave (light orange) diffuses from this location. This can led to engagement of redox signaling pathways (e.g. Src kinase), and to biological responses of interest in redox biology. (C) When large amounts of 1O2 (red) are generated, either by a strong light flux or by a long exposure time, a general cytoplasmic oxidation sets in (deep orange), which signals for pathological responses. In this scheme, the irradiated cell is undergoing apoptosis. The nucleus also shows a turn towards oxidative conditions (light orange), the nuclear membrane has dissolved, and chromatin appears condensed and fragmented (karyorrhexis). Adapted from [33], [210].
5.3. Light sources
The most adequate light sources for direct optical excitation of 1O2 are lasers. Several are the arguments underpinning this statement. Lasers produce quite monochromatic light. This means they emit almost pure colors (i.e. at a single wavelength). As O2 absorbs in such narrow bands it is reasonable to pump also with a narrow wavelength source, to avoid wasting most of the light not “tuned” to O2. Lasers can be cw or pulsed. Both kinds can provide very useful results by direct O2 excitation. As mentioned previously, for most redox biological experiments cw lasers are better suited as non-linear optical effects are avoided. Some lasers are tunable (e.g. Ti:sapphire) which is even more suited because experiments both at actinic and control wavelengths can be accomplished in a simpler setup. These light sources can be very powerful emitting powers from several watts for cw lasers to gigawatts/terawatts for fs pulsed ones. These optical powers, combined with monochromaticity, ensure that shorter times are necessary to achieve biological responses. For microscopic studies (e.g. single cell experiments) the coherence of laser light allow for focusing nearly to the diffraction limit. For visible wavelengths, this puts the limit below a micron under optimal conditions.
Many different laser types have been employed to directly pump monomol and dimol O2 bands: Nd:YAG and Nd-based [29], [30], [122], [123], [124], [125], [126], [127], [128], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], Yb-based [31], He-Ne [29], [99], [108], [123], [141], [143], [144], [177], [178], [179], [180], [181], semiconductor (diode) [27], [108], [109], [114], [119], [120], [137], [146], [148], [149], [150], [151], [152], [153], [154], [168], Raman-shifted [23], [24], [25], [26], [28], [115], dye-based [174], [185], alexandrite (Cr:BeAl2O4) [108], and forsterite (Cr:Mg2SiO4) [109] to name the most common ones (more examples can be found in each wavelength subsection above). Visible excitation of O2 has the drawback of overlapping with endogenous cellular chromophores, like cytochromes, porphyrins, certain cofactors (like flavin- and nicotinamide-containing metabolites), etc [170]. Therefore NIR excitation (690–1270 nm) is preferable to avoid complex results. However, wavelengths above ~950 nm excite directly the vibrational overtones of H2O which leads to sample heating [121], [212]. This can be adequately dealt with doing the right experimental controls. But, given that some excitation wavelengths (690–920) fall between these two spectral regions (the so-called optical biological/therapeutic window [15], [68], [69]), it is reasonable to suggest their use for redox biology studies. Furthermore, absorption coefficients for 810 and 690 nm are very low, which leaves 760 and 920 nm as the best suited bands for these kind of studies. Absorption at 760 nm is stronger than at 920 nm [103], which explains that some recent reports have been done at this wavelength [32], [33], [34].
Other light sources have been shown to directly photoexcite O2, but different features like polychromaticity, low power, incoherence, etc. make them much less suitable than lasers for the topic of this review. LEDs are probably the second best option to obtain 1O2. Recent publications have reported on the feasibility of this approach [146], [213], [214], [215]. It will be interesting to see the practical importance of these light sources, although they can be adequate for low 1O2 dose studies like those studying biological stimulation [146]. As a historical remark Evans was able to produce 1O2 by optical excitation employing halogens lamps and narrow-band filters to isolate O2 excitation bands from the blackbody lamp spectrum [99].
5.4. Fluorescence microscopy
Light has an assessing, relatively passive role in fluorescence microscopy by exciting some fluorophore of interest. It turns out, however, that it can excite many other unwanted chromophores. Given the narrow and weak absorption bands of O2 in the visible, the concerns should be towards organic molecules capable of absorption, not to oxygen. As such, most undesirable oxidative action occurring in fluorescence microscopy is due the classical photodynamic effect or other photochemical reactions. However, currently it is possible to excite in the far red/NIR and record the fluorescent emission in the NIR. Thus, fluorophores excited around 690 nm and 760 nm should be discarded in favor of others or changing the excitation wavelengths.
Non-linear excitation fluorescence microscopy makes much common use of NIR wavelengths [216], [217]. Following the same arguments presented above, O2 absorption bands should be avoided to preserve sample integrity. In this particular field excitation with Ti:sapphire pulsed lasers is very common. Care must be taken not to excite at ~760 nm, ~810 nm or ~920 nm. Additionally, it can be a good idea to use some kind of antioxidant compound (1,4-diazabicyclo[2.2.2]octane –DABCO- is a useful option) or antioxidant fluorescence medium to quench ROS action during sample imaging. This will depend on viability issues, but it is worth assessing its usefulness. This also applies to linear excitation fluorescence microscopy.
Finally it can be interesting to try to detect 1O2 phosphorescence at ~1270 nm after direct excitation at 760 nm in living samples [10], [32], [37], [46]. This is difficult because of the low amount of 1O2 produced in the first place and the very low rate of light emission, especially in the reduced environment that defines living systems. The probability of solvent quenching by H2O or chemical reaction leave small margin for light emission. Photon detection at 1270 nm requires dedicated equipment but this has been already proved with a classical photodynamic excitation of 1O2 in living cells [47].
5.5. Optical traps
As repeatedly mentioned in Section 4 a significant part of the damage observed with optical tweezers/traps can be assigned to 1O2 directly excited by NIR light [30], [135], [139], [163], [164]. The different action spectra reported for deleterious biological action with optical traps clearly fits to the 3O2 absorption spectrum [136], [137], [138], [142], [145], [161], [218]. Therefore, researchers should refrain of using those wavelengths that fall within the O2 absorption bands (i.e. 760 nm, 810 nm, 920 nm, and 1065 nm). Use of antioxidants and quenchers can be useful ways of minimizing the effect of any 1O2 eventually produced, as long as they do not have negative effects on the samples or these are dealt with adequately with proper controls. At least in one case pre-irradiation at 632.8 nm was employed to “prime” cells for later exposure to 1064 nm light. Cells exposed to 632.8 nm endured better the subsequent irradiation to 1064 nm [141]. Alternatively, optical tweezers working with actinic light can be put to use to assess non-adherent cell redox responses. The cell can be trapped and exposed to 1O2 at the same time. An interesting possibility is to study biomechanical aspects, like cell membrane fluidity or intracellular viscosity during trapping, as the optical tweezers can induce controlled mechanical forces on the cell. Changes in these properties can be correlated to the light flux and the 1O2 dose. Certainly, large viscosity changes have already been reported in relation to photodynamic treatments of cultured cells [219].
5.6. Thermal aspects
Finally, some comments on thermal aspects seem appropriate. Due to the very high light intensities and/or focusing, it is reasonable to assume that very high thermal loads should happen under most experimental situations described. However, absorption by H2O is relatively low below 1300 nm and almost negligible below 950 nm (biological window) [121]. Unless some exogenous strongly absorbing compound/structure (e.g. nanoparticles) has been introduced in the studied system, water should be the main concern in regards to thermal side effects. This issue has been both theoretically modelled and experimentally measured for different wavelengths and situations [220], [221], [222]. Consensus is that the temperature increase in the focal plane is around 1 °C for every 100 mW of light power at 1064 nm. Making use of shorter wavelengths (760 or 920 nm) should reduce the thermal load by at least one order of magnitude. Although heat-driven damage seems not to be a factor of concern, assuming the previous arguments, it is nevertheless important to be aware that some biological effects can have a thermal origin in some redox experiments using light [223], [224], [225], [226], [227], [228], [229]. Under certain circumstances, ironically, thermal damage can be an asset. For example, Yusupov et al. reported on the efficient clinical treatment of skin tumors making use of a laser emitting at 1262 nm [23]. The authors assign the main toxic action on the lesions to the production of 1O2, but thermal synergy could not be discarded. Therefore, a combination of oxidative damage and thermal stress can significantly enhance the clinical outcome of certain phototherapies [230].
6. Summary
Direct optical excitation of O2 for redox biology seems a promising and very productive research area. Definitively, it is a highly interdisciplinary field, which requires inputs from redox biology, biophotonics, physical chemistry, physics, etc. At the same time, it can help respond questions and define new parameters, with the potential to shed light upon many biomedical topics, from ROS-induced pathologies to regenerative processes. It is one of the goals of this review to have introduced the general methodology to the broad audience of researchers in redox biology and photobiology; and, hopefully, to have sparked interest in them about its potentials and further developments.
Conflict of interest
The author declares no conflict of interest.
Acknowledgements
I would like to acknowledge very fruitful discussions, long-term support and critic feedback from Prof. Juan Carlos Stockert, Dr. Begoña López, Dr. Elisa Carrasco and Antonio Romero. Additional support and feedback has been provided by Prof. Peter R. Ogilby and Dr. Thomas Breitenbach. I am thankful to Mikkel Bregnhøj and Michael Westberg for stimulating conversations on this topic too. Experimental work with cw laser sources is acknowledged to Prof. Daniel Jaque and Dr. Patricia Haro. Very constructive comments and suggestions from the reviewers are acknowledged. Prof. Arturo Morales is acknowledged for logistic support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lane N. Oxford University Press; New York USA: 2002. Oxygen: The Molecule That Made the World. [Google Scholar]
- 2.Pryor W.A., Houk K.N., Foote C.S., Fukuto J.M., Ignarro L.J. Free radical biology and medicine: it's a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 3.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 4.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweitzer C., Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- 7.Slanger T.G., Copeland R.A. Energetic oxygen in the upper atmosphere and the laboratory. Chem. Rev. 2003;103:4731–4765. doi: 10.1021/cr0205311. [DOI] [PubMed] [Google Scholar]
- 8.Minaev B.F. Electronic mechanisms of molecular oxygen activation. Russ. Chem. Rev. 2007;76:988–1010. [Google Scholar]
- 9.Krasnovsky A.A. Primary mechanisms of photoactivation of molecular oxygen. Hist. Dev. Mod. Status Res., Biochem. 2007;72:1065–1080. doi: 10.1134/s0006297907100057. [DOI] [PubMed] [Google Scholar]
- 10.Ogilby P.R. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 2010;39:3181–3209. doi: 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- 11.Dolmans D.E.J.G.J., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 12.Wilson B.C., Patterson M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008;53:R61–R109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 13.Robertson C.A., Hawkins Evans D., Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B: Biol. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W. Photodynamic therapy of cancer: an update. CA Cancer. J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dąbrowski J.M., Arnaut L.G. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem. Photobiol. Sci. 2015;14:1765–1780. doi: 10.1039/c5pp00132c. [DOI] [PubMed] [Google Scholar]
- 16.Klotz L.O., Kröncke K.D., Sies H. Singlet oxygen-induced signaling effects in mammalian cells. Photochem. Photobiol. Sci. 2003;2:88–94. doi: 10.1039/b210750c. [DOI] [PubMed] [Google Scholar]
- 17.Onyango A.N. Endogenous generation of singlet oxygen and ozone in human and animal tissues: mechanisms, biological significance, and influence of dietary components. Oxid. Med. Cell. Longev. 2016:2398573. doi: 10.1155/2016/2398573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long M.J.C., Poganik J.R., Ghosh S., Aye Y. Subcellular redox targeting: bridging in Vitro and in Vivo chemical biology. ACS Chem. Biol. 2017;12:586–600. doi: 10.1021/acschembio.6b01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boonstra J., Post J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Kemp M., Go Y.M., Jones D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Rad. Biol. Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blázquez-Castro A., Breitenbach T., Ogilby P.R. Singlet oxygen and ROS in a new light: low-dose subcellular photodynamic treatment enhances proliferation at the single cell level. Photochem. Photobiol. Sci. 2014;13:1235–1240. doi: 10.1039/c4pp00113c. [DOI] [PubMed] [Google Scholar]
- 22.F. Anquez, A. Sivéry, I. El Yazidi-Belkoura, J. Zemmouri, P. Suret, et al., Chapter 4: Production of singlet oxygen by direct photoactivation of molecular oxygen, in: S. Nonell, C. Flors (Eds.), Singlet Oxygen: Applications in Biosciences and Nanosciences, Volume 1, Ch. 4, Comprehensive Series in Photochemical & Photobiological Sciences, Royal Society of Chemistry, 2016. DOI: https://dx.doi.org/10.1039/9781782622208-00075.
- 23.Yusupov A.S., Goncharov S.E., Zalevskii I.D., Paramonov V.M., Kurkov A.S. Raman fiber laser for the drug-free photodynamic therapy. Laser Phys. 2010;20:357–359. [Google Scholar]
- 24.Anquez F., El Yazidi-Belkoura I., Randoux S., Suret P., Courtade E. Cancerous cell death from sensitizer free photoactivation of singlet oxygen. Photochem. Photobiol. 2012;88:167–174. doi: 10.1111/j.1751-1097.2011.01028.x. [DOI] [PubMed] [Google Scholar]
- 25.Detty M.R. Direct 1270 nm irradiation as an alternative to photosensitized generation of singlet oxygen to induce cell death. Photochem. Photobiol. 2012;88:2–4. doi: 10.1111/j.1751-1097.2011.01047.x. [DOI] [PubMed] [Google Scholar]
- 26.Gening T.P., Voronova O.S., Dolgova D.R., Abakumova T.V., Zolotovskii I.O. Analysis of the efficiency of using 1265-nm cw laser radiation for initiating oxidative stress in the tissue of a solid malignant tumour. Quant. Electron. 2012;42:805–807. [Google Scholar]
- 27.Sokolovski S.G., Zolotovskaya S.A., Goltsov A., Pourreyron C., South A.P. Infrared laser pulse triggers increased singlet oxygen production in tumour cells. Sci. Rep. 2013;3:3484. doi: 10.1038/srep03484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saenko Y.V., Glushchenko E.S., Zolotovskii I.O., Sholokhov E., Kurkov A. Mitochondrial dependent oxidative stress in cell culture induced by laser radiation at 1265 nm. Lasers Med. Sci. 2016;31:405–413. doi: 10.1007/s10103-015-1861-z. [DOI] [PubMed] [Google Scholar]
- 29.Houreld N.N., Abrahamse H. Cellular damage in diabetic wounded fibroblast cells following phototherapy at 632.8, 830, and 1064 nm. Laser Chem. 2007:80536. [Google Scholar]
- 30.Landry M.P., McCall P.M., Qi Z., Chemla Y.R. Characterization of photoactivated singlet oxygen damage in single-molecule optical trap experiments. Biophys. J. 2009;97:2128–2136. doi: 10.1016/j.bpj.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aabo T., Perch-Nielsen I.R., Dam J.S., Palima D.Z., Siegumfeldt H. Effect of long- and short-term exposure to laser light at 1070 nm on growth of Saccharomyces cerevisiae. J. Biomed. Opt. 2010;15:041505. doi: 10.1117/1.3430731. [DOI] [PubMed] [Google Scholar]
- 32.Bregnhøj M., Blázquez-Castro A., Westberg M., Breitenbach T., Ogilby P.R. Direct 765 nm optical excitation of molecular oxygen in solution and in single mammalian cells. J. Phys. Chem. B. 2015;119:5422–5429. doi: 10.1021/acs.jpcb.5b01727. [DOI] [PubMed] [Google Scholar]
- 33.Westberg M., Bregnhøj M., Blázquez-Castro A., Breitenbach T., Etzerodt M. Control of singlet oxygen production in experiments performed on single mammalian cells. J. Photochem. Photobiol. A: Chem. 2016;321:297–308. [Google Scholar]
- 34.Westberg M., Bregnhøj M., Banerjee C., Blázquez-Castro A., Breitenbach T. Exerting better control and specificity with singlet oxygen experiments in live mammalian cells. Methods. 2016;109:81–91. doi: 10.1016/j.ymeth.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Kasha M., Khan A.U. The physics, chemistry, and biology of singlet molecular oxygen. Ann. N.Y. Acad. Sci. 1970:5–23. [Google Scholar]
- 36.Kearns D.R. Physical and chemical properties of singlet molecular oxygen. Chem. Rev. 1971;71:395–427. [Google Scholar]
- 37.Weldon D., Poulsen T.D., Mikkelsen K.V., Ogilby P.R. Singlet sigma: the “other” singlet oxygen in solution. Photochem. Photobiol. 1999;70:369–379. [Google Scholar]
- 38.Clennan E.L., Pace A. Advances in singlet oxygen chemistry. Tetrahedron. 2005;61:6665–6691. [Google Scholar]
- 39.Romero N.A., Nicewicz D.A. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 40.Ghogare A.A., Greer A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 2016;116:9994–10034. doi: 10.1021/acs.chemrev.5b00726. [DOI] [PubMed] [Google Scholar]
- 41.Blázquez-Castro A., Carrasco E., Calvo M.I., Jaén P., Stockert J.C. Protoporphyrin IX-dependent photodynamic production of endogenous ROS stimulates cell proliferation. Eur. J. Cell Biol. 2012;91:216–223. doi: 10.1016/j.ejcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco E., Calvo M.I., Blázquez-Castro A., Vecchio D., Zamarron A. Photoactivation of ROS production In Situ transiently activates cell proliferation in mouse skin and in the hair follicle stem cell niche promoting hair growth and wound healing. J. Invest. Dermatol. 2015;135:2611–2622. doi: 10.1038/jid.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrasco E., Blázquez-Castro A., Calvo M.I., Juarranz A., Espada J. Switching on a transient endogenous ROS production in mammalian cells and tissues. Methods. 2016;109:180–189. doi: 10.1016/j.ymeth.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 44.Minaev B.F. Spin-orbit coupling mechanism of singlet oxygen a1Δg quenching by solvent vibrations. Chem. Phys. 2017;483–484:84–95. [Google Scholar]
- 45.Bregnhøj M., Ogilby P.R. Effect of solvent on the O2(a1Δg) → O2(b1Σg+) absorption coefficient. J. Phys. Chem. A. 2015;119:9236–9243. doi: 10.1021/acs.jpca.5b05131. [DOI] [PubMed] [Google Scholar]
- 46.Bregnhøj M., Krægpøth M.V., Sørensen R.J., Westberg M., Ogilby P.R. Solvent and heavy-atom effects on the O2(X3Σg−) → O2(b1Σg+) absorption transition. J. Phys. Chem. A. 2016;120:8285–8296. doi: 10.1021/acs.jpca.6b08035. [DOI] [PubMed] [Google Scholar]
- 47.Skovsen E., Snyder J.W., Lambert J.D.C., Ogilby P.R. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B. 2005;109:8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 48.Redmond R.W., Kochevar I.E. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006;82:1178–1186. doi: 10.1562/2006-04-14-IR-874. [DOI] [PubMed] [Google Scholar]
- 49.Wojtovich A.P., Foster T.H. Optogenetic control of ROS production. Redox Biol. 2014;2:368–376. doi: 10.1016/j.redox.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao L., Liu R., Gao F., Wang Y., Jiang X. Plasmon-mediated generation of reactive oxygen species from near-infrared light excited gold nanocages for photodynamic therapy in Vitro. ACS Nano. 2014;8:7260–7271. doi: 10.1021/nn502325j. [DOI] [PubMed] [Google Scholar]
- 51.Smith B.E., Roder P.B., Hanson J.L., Manandhar S., Devaraj A. Singlet-oxygen generation from individual semiconducting and metallic nanostructures during near-infrared laser trapping. ACS Photonics. 2015;2:559–564. [Google Scholar]
- 52.Brongersma M.L., Halas N.J., Nordlander P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015;10:25–34. doi: 10.1038/nnano.2014.311. [DOI] [PubMed] [Google Scholar]
- 53.Mano C.M., Prado F.M., Massari J., Ronsein G.E., Martinez G.R. Excited singlet molecular O2 (1Δg) is generated enzymatically from excited carbonyls in the dark. Sci. Rep. 2014;4:5938. doi: 10.1038/srep05938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinbeck M.J., Khan A.U., Karnovsky M.J. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J. Biol. Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- 55.Winterbourn C.C., Kettle A.J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 56.Turro N.J., Chow M.F., Rigaudy J. Mechanism of thermolysis of endoperoxides of aromatic compounds. Activation parameters, magnetic field, and magnetic isotope effects. J. Am. Chem. Soc. 1981;103:7218–7224. [Google Scholar]
- 57.Aubry J.M., Pierlot C., Rigaudy J., Schmidt R. Reversible binding of oxygen to aromatic compounds. Acc. Chem. Res. 2003;36:668–675. doi: 10.1021/ar010086g. [DOI] [PubMed] [Google Scholar]
- 58.Grether-Beck S., Olaizola-Horn S., Schmitt H., Grewe M., Jahnke A. Activation of transcription factor AP-2 mediates UVA radiation- and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14586–14591. doi: 10.1073/pnas.93.25.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.C. Pierlot, J.M. Aubry, K. Briviba, H. Sies, P. Di Mascio, Naphthalene endoperoxides as generators of singlet oxygen in biological media, Chapter 1, pp. 3–20, in: Methods in Enzymology, Vol. 319 Singlet Oxygen, UV-A, and Ozone, Academic Press, 2000. DOI: https://dx.doi.org/10.1039/9781782622208-0007510.1016/S0076-6879(00)19003-2. [DOI] [PubMed]
- 60.K. Krumova, G. Cosa, Chapter 1: Overview of reactive oxygen species, in: S. Nonell, C. Flors (Eds.) Singlet Oxygen: Applications in Biosciences and Nanosciences, Volume 1, Ch. 1, Comprehensive Series in Photochemical & Photobiological Sciences, Royal Society of Chemistry, 2016. DOI: https://dx.doi.org/10.1039/9781782622208-0007510.1039/9781782622208-00001.
- 61.Kramarenko G.G., Hummel S.G., Martin S.M., Buettner G.R. Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochem. Photobiol. 2006;82:1634–1637. doi: 10.1562/2006-01-12-RN-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayyan M., Hashim M.A., AlNashef I.M. Superoxide ion: generation and chemical implications. Chem. Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 63.Peters G., Rodgers M.A.J. Single-electron transfer from NADH analogues to singlet oxygen. Biochim. Biophys. Acta Bioenerg. 1981;637:43–52. doi: 10.1016/0005-2728(81)90208-5. [DOI] [PubMed] [Google Scholar]
- 64.Saito I., Matsuura T., Inoue K. Formation of superoxide ion via one-electron transfer from electron donors to singlet oxygen. J. Am. Chem. Soc. 1983;105:3200–3206. [Google Scholar]
- 65.Wefers H., Sies H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur. J. Biochem. 1983;137:29–36. doi: 10.1111/j.1432-1033.1983.tb07791.x. [DOI] [PubMed] [Google Scholar]
- 66.Cosso R.G., Turim J., Nantes I.L., Almeida A.M., Di Mascio P. Mitochondrial permeability transition induced by chemically generated singlet oxygen. J. Bioenerg. Biomembr. 2002;34:157–163. doi: 10.1023/a:1016075218162. [DOI] [PubMed] [Google Scholar]
- 67.von Monfort C., Sharov V.S., Metzger S., Schöneich C., Sies H. Singlet oxygen inactivates protein tyrosine phosphatase-1B by oxidation of the active site cysteine. Biol. Chem. 2006;387:1399–1404. doi: 10.1515/BC.2006.175. [DOI] [PubMed] [Google Scholar]
- 68.Bacellar I.O.L., Tsubone T.M., Pavani C., Baptista M.S. Photodynamic efficiency: from molecular photochemistry to cell death. Int. J. Mol. Sci. 2015;16:20523–20559. doi: 10.3390/ijms160920523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benov L. Photodynamic therapy: current status and future directions. Med. Princ. Pract. 2015;24:14–28. doi: 10.1159/000362416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graindorge D., Martineau S., Machon C., Arnoux P., Guitton J. Singlet oxygen-mediated oxidation during UVA radiation alters the dynamic of genomic DNA replication. PLoS One. 2015;10:e0140645. doi: 10.1371/journal.pone.0140645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao J.G., Shih A., Simon M. The role of singlet oxygen in the UVA-induced bystander effect on dermal fibroblasts. FASEB J. 2011;25:528.5. [Google Scholar]
- 72.Piette J. Signalling pathway activation by photodynamic therapy: nf-κb at the crossroad between oncology and immunology. Photochem. Photobiol. Sci. 2015;14:1510–1517. doi: 10.1039/c4pp00465e. [DOI] [PubMed] [Google Scholar]
- 73.Berneburg M., Grether-Beck S., Kürten V., Ruzicka T., Briviba K. Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J. Biol. Chem. 1999;274:15345–15349. doi: 10.1074/jbc.274.22.15345. [DOI] [PubMed] [Google Scholar]
- 74.Ravanat J.L., Di Mascio P., Martinez G.R., Medeiros M.H.G., Cadet J. Singlet oxygen induces oxidation of cellular DNA. J. Biol. Chem. 2000;275:40601–40604. doi: 10.1074/jbc.M006681200. [DOI] [PubMed] [Google Scholar]
- 75.Ravanat J.L., Sauvaigo S., Caillat S., Martinez G.R., Medeiros M.H. Singlet oxygen-mediated damage to cellular DNA determined by the comet assay associated with DNA repair enzymes. Biol. Chem. 2004;385:17–20. doi: 10.1515/BC.2004.003. [DOI] [PubMed] [Google Scholar]
- 76.Kessel D. Autophagic death probed by photodynamic therapy. Autophagy. 2015;11:1941–1943. doi: 10.1080/15548627.2015.1078960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattison D.I., Rahmanto A.S., Davies M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012;11:38–53. doi: 10.1039/c1pp05164d. [DOI] [PubMed] [Google Scholar]
- 78.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies M.J. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 80.J. Cadet, T. Douki, J.L. Ravanat, P. Di Mascio, Chapter 20: Reactions of singlet oxygen with nucleic acids, in: S. Nonell, C. Flors (Eds.) Singlet Oxygen: Applications in Biosciences and Nanosciences, Volume 1, Ch. 20, Comprehensive Series in Photochemical & Photobiological Sciences, Royal Society of Chemistry, 2016. DOI: https://dx.doi.org/10.1039/9781782622208-00393.
- 81.Yasui M., Kanemaru Y., Kamoshita N., Suzuki T., Arakawa T. Tracing the fates of site-specifically introduced DNA adducts in the human genome. DNA Repair. 2014;15:11–20. doi: 10.1016/j.dnarep.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Li J., Braganza A., Sobol R.W. Base excision repair facilitates a functional relationship between guanine oxidation and histone demethylation. Antioxid. Redox Signal. 2013;18:2429–2443. doi: 10.1089/ars.2012.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.A.W. Girotti, W. Korytowski, Chapter 21: Reactions of singlet oxygen with membrane lipids: lipid hydroperoxide generation, translocation, reductive turnover, and signaling activity, in: S. Nonell, C. Flors (Eds.) Singlet Oxygen: Applications in Biosciences and Nanosciences, Volume 1, Ch. 21, Comprehensive Series in Photochemical & Photobiological Sciences, Royal Society of Chemistry, 2016. DOI: https://dx.doi.org/10.1039/9781782622208-00409.
- 84.Girotti A.W. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B: Biol. 2001;63:103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 85.Miyamoto S., Martínez G.R., Medeiros M.H.G., Di Mascio P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B: Biol. 2014;139:24–33. doi: 10.1016/j.jphotobiol.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 86.Girotti A.W. Translocation as a means of disseminating lipid hydroperoxide-induced oxidative damage and effector action. Free Radic. Biol. Med. 2008;44:956–968. doi: 10.1016/j.freeradbiomed.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong M., Xu A., Zhou H., Wu L., Randers-Pehrson G. Mechanism of genotoxicity induced by targeted cytoplasmic irradiation. Br. J. Cancer. 2010;103:1263–1268. doi: 10.1038/sj.bjc.6605888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong H., Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidat. Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ronsein G.E., De Oliveira M.C.B., Medeiros M.H.G., Miyamoto S., Di Mascio P. DNA strand breaks and base modifications induced by cholesterol hydroperoxides. Free Radic. Res. 2011;45:266–275. doi: 10.3109/10715762.2010.524215. [DOI] [PubMed] [Google Scholar]
- 91.Wlaschek M., Briviba K., Stricklin G.P., Sies H., Scharffetter-Kochanek K. Singlet oxygen may mediate the ultraviolet a-induced synthesis of interstitial collagenase. J. Invest. Dermatol. 1995;104:194–198. doi: 10.1111/1523-1747.ep12612751. [DOI] [PubMed] [Google Scholar]
- 92.Wlaschek M., Wenk J., Brenneisen P., Briviba K., Schwarz A. Singlet oxygen is an early intermediate in cytokine-dependent ultraviolet-A induction of interstitial collagenase in human dermal fibroblasts in vitro. FEBS Lett. 1997;413:239–242. doi: 10.1016/s0014-5793(97)00919-8. [DOI] [PubMed] [Google Scholar]
- 93.Klotz L.O., Pellieux C., Briviba K., Pierlot C., Aubry J.M. Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. FEBS J. 1999;260:917–922. doi: 10.1046/j.1432-1327.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 94.Stief T.W. The physiology and pharmacology of singlet oxygen. Med. Hypotheses. 2003;60:567–572. doi: 10.1016/S0306-9877(03)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Girotti A.W., Kriska T. Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid. Redox Signal. 2004;6:301–310. doi: 10.1089/152308604322899369. [DOI] [PubMed] [Google Scholar]
- 96.P.R. Ogilby, M.K. Kuimova, Chapter 34: Singlet oxygen in mammalian cells, in Singlet Oxygen : Applications in Biosciences and Nanosciences (Ed. S. Nonell, C. Flors), Volume 2, Ch. 34, Comprehensive Series in Photochemical & Photobiological Sciences, Royal Society of Chemistry (2016) DOI: https://dx.doi.org/10.1039/9781782626992-00169.
- 97.Giannoni E., Taddei M.L., Chiarugi P. Src redox regulation: again in the front line. Free Radic. Biol. Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 98.Herzberg G. Molecular Spectra and Molecular Structure: I. Spectra of Diatomic Molecules. 2nd edition. Van Nostrand Reinhold Company-Prentice Hall; New York: 1950. [Google Scholar]
- 99.Evans D.F. Oxidation by photochemically produced singlet states of oxygen. J. Chem. Soc. D. 1969:367–368. [Google Scholar]
- 100.Matheson I.B.C., Lee J. Comparison of the pressure dependencies of the visible and infrared electronic absorption spectra of oxygen in gas and in Freon solution. Chem. Phys. Lett. 1971;8:173–176. [Google Scholar]
- 101.Huestis D.L., Black G., Edelstein S.A., Sharpless R.L. Fluorescence and quenching of O2(1Δg) and [O2(1Δg)]2 in liquid oxygen. J. Chem. Phys. 1974;60:4471–4474. [Google Scholar]
- 102.Cooper P.D., Johnson R.E., Quickenden T.I. A review of possible optical absorption features of oxygen molecules in the icy surfaces of outer solar system bodies. Planet. Space Sci. 2003;51:183–192. [Google Scholar]
- 103.Jockusch S., Turro N.J., Thompson E.K., Gouterman M., Callis J.B. Singlet molecular oxygen by direct excitation. Photochem. Photobiol. Sci. 2008;7:235–239. doi: 10.1039/b714286b. [DOI] [PubMed] [Google Scholar]
- 104.Thalman R., Volkamer R. Temperature dependent absorption cross-sections of O2–O2 collision pairs between 340 and 630 nm and at atmospherically relevant pressure. Phys. Chem. Chem. Phys. 2013;15:15371. doi: 10.1039/c3cp50968k. [DOI] [PubMed] [Google Scholar]
- 105.Krasnovsky A.A., Kozlov A.S., Roumbal Y. Photochemical investigation of the IR absorption bands of molecular oxygen in organic and aqueous environment. Photochem. Photobiol. Sci. 2012;11:988–997. doi: 10.1039/c2pp05350k. [DOI] [PubMed] [Google Scholar]
- 106.Krasnovsky A.A., Kozlov A.S. New approach to measurement of IR absorption spectra of dissolved oxygen molecules based on photochemical activity of oxygen upon direct laser excitation. Biophysics. 2014;59:199–205. [PubMed] [Google Scholar]
- 107.Krasnovsky A.A., Kozlov A.S. Photonics of dissolved oxygen molecules. Comparison of the rates of direct and photosensitized excitation of oxygen and reevaluation of the oxygen absorption coefficients. J. Photochem. Photobiol. A: Chem. 2016;329:167–174. [Google Scholar]
- 108.Zakharov S.D., Ivanov A.V. Light-oxygen effect in cells and its potential applications in tumour therapy (review) Quant. Electron. 1999;29:1031–1053. [Google Scholar]
- 109.Krasnovsky A.A., Drozdova N.N., Ivanov A.V., Ambartsumian R.V. Activation of molecular oxygen by infrared laser radiation in pigment-free aerobic systems. Biochemistry. 2003;68:963–966. doi: 10.1023/a:1026052310563. [DOI] [PubMed] [Google Scholar]
- 110.Krasnovsky A.A., Ambartzumian R.V. Tetracene oxygenation caused by infrared excitation of molecular oxygen in air-saturated solutions: the photoreaction action spectrum and spectroscopic parameters of the 1Δg ← 3Σ-g transition in oxygen molecules. Chem. Phys. Lett. 2004;400:531–535. [Google Scholar]
- 111.Krasnovsky A.A., Drozdova N.N., Roumbal Y.V., Ivanov A.V., Ambartzumian R.V. Biophotonics of molecular oxygen: activation efficiencies upon direct and photosensitized excitation. Chin. Opt. Lett. 2005;3:S1–S4. [Google Scholar]
- 112.Krasnovsky A.A., Roumbal Y.V., Ivanov A.V., Ambartzumian R.V. Solvent dependence of the steady-state rate of 1O2 generation upon excitation of dissolved oxygen by cw 1267 nm laser radiation in air-saturated solutions: estimates of the absorbance and molar absorption coefficients of oxygen at the excitation wavelength. Chem. Phys. Lett. 2006;430:260–264. [Google Scholar]
- 113.Krasnovsky A.A. Luminescence and photochemical studies of singlet oxygen photonics. J. Photochem. Photobiol. A: Chem. 2008;196:210–218. [Google Scholar]
- 114.Krasnovsky A.A., Roumbal Y.V., Strizhakov A.A. Rates of 1O2 (1Δg) production upon direct excitation of molecular oxygen by 1270 nm laser radiation in air-saturated alcohols and micellar aqueous dispersions. Chem. Phys. Lett. 2008;458:195–199. [Google Scholar]
- 115.Anquez F., Courtade E., Sivéry A., Suret P., Randoux S. A high-power tunable Raman fiber ring laser for the investigation of singlet oxygen production from direct laser excitation around 1270 nm. Opt. Express. 2010;22:22928–22936. doi: 10.1364/OE.18.022928. [DOI] [PubMed] [Google Scholar]
- 116.Anquez F., El Yazidi-Belkoura I., Suret P., Randoux S., Courtade E. Cell death induced by direct laser activation of singlet oxygen at 1270 nm. Laser Phys. 2013;23:025601. [Google Scholar]
- 117.Sivéry A., Anquez F., Pierlot C., Aubry J.M., Courtade E. Singlet oxygen (1O2) generation upon 1270 nm laser irradiation of ground state oxygen (3O2) dissolved in organic solvents: simultaneous and independent determination of 1O2 production rate and reactivity with chemical traps. Chem. Phys. Lett. 2013;555:252–257. [Google Scholar]
- 118.Sivéry A., Barras A., Boukherroub R., Pierlot C., Aubry J.M. Production rate and reactivity of singlet oxygen 1O2(1Δg) directly photoactivated at 1270 nm in lipid nanocapsules dispersed in water. J. Phys. Chem. C. 2014;118:2885–2893. [Google Scholar]
- 119.S.G. Sokolovski, A. Goltsov, E.U. Rafailov, Modelling the hypersensitivity of cancer cells to infra-red laser pulse: breaking ROS defence machinery, in: Proceedings SPIE 8568, Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XXII, 85680E. DOI: https://dx.doi.org/10.1117/12.2001715, 2013.
- 120.E.U. Rafailov, K.S. Litvinova, S.G. Sokolovski, Towards novel compact laser sources for non-invasive diagnostics and treatment, in: Proceedings of SPIE Vol. 9550, Biosensing and Nanomedicine VIII 95500G. DOI: https://dx.doi.org/10.1117/12.2193777, 2015.
- 121.Hale G.M., Querry M.R. Optical constants of water in the 200-nm to 200-µm wavelength region. Appl. Opt. 1973;12:555–563. doi: 10.1364/AO.12.000555. [DOI] [PubMed] [Google Scholar]
- 122.Matheson I.B.C., Lee J. Reaction of chemical acceptors with singlet oxygen produced by direct laser excitation. Chem. Phys. Lett. 1970;7:475–476. [Google Scholar]
- 123.Evans D.F., Tucker J.N. Reactivity of the (1Δg)2 and 1Δg states of oxygen produced by direct laser excitation. J. Chem. Soc. Faraday Trans. 1976;2:1661–1666. [Google Scholar]
- 124.Matheson I.B.C. The absolute value of the reaction rate constant of bilirubin with singlet oxygen in D2O. Photochem. Photobiol. 1979;29:875–878. [Google Scholar]
- 125.Skuja L., Güttler B. Detection of interstitial oxygen molecules in SiO2 glass by a direct photoexcitation of the infrared luminescence of singlet O2. Phys. Rev. Lett. 1996;77:2093–2096. doi: 10.1103/PhysRevLett.77.2093. [DOI] [PubMed] [Google Scholar]
- 126.Matheson I.B.C., Lee J. Observation of the singlet oxygen dimol emission from neodymium laser pumped oxygen in the gas phase and in 1,1,2-trichlorotrifluoroethane solution. Chem. Phys. Lett. 1974;27:355–358. [Google Scholar]
- 127.Protz R., Maier M. Laser induced fluorescence and relaxation of singlet molecular oxygen the liquid phase. J. Chem. Phys. 1980;73:5464–5467. [Google Scholar]
- 128.Yamagishi A., Ohta T., Konno J., Inaba H. Visible fluorescence of liquid oxygen excited by a Q-switched Nd:YAG laser. J. Opt. Soc. Am. 1981;71:1197–1201. [Google Scholar]
- 129.Zapol´skiĭ O.B., Igoshin V.I., Oraevskiĭ A.N. Feasibility of lasing in collision-induced dipole transitions of molecular oxygen. JETP Lett. 1975;21:529–532. [Google Scholar]
- 130.B. Zhadanov, T.L. Henshaw, K. Hobbs, D.K. Neumann, Generation of high densities of gas phase O2(a1Δ) from optical pumping of liquid oxygen, in: Proceedings of Conference on Lasers and Electro-Optics, 2003. CLEO '03, 2003.
- 131.R.R. Reibel, J.K. Brasseur, D.K. Neumann, Optically pumped liquid oxygen jet waveguides for production of singlet delta oxygen, in: Proceedings of Conference on Lasers and Electro-Optics, 2004. CLEO '04, 2004.
- 132.Huestis D.L. Challenges for lasers in liquid oxygen. Chem. Phys. Lett. 2005;411:108–110. [Google Scholar]
- 133.Kumar A., Rajesh R., Singhal G., Tyagi R.K. Analysis of various optical pumping schemes for liquid oxygen lasers. Appl. Phys. B. 2007;89:385–394. [Google Scholar]
- 134.Matheson I.B.C., Lee J. Chemical reaction rates of amino acids with singlet oxygen. Photochem. Photobiol. 1979;29:879–881. [Google Scholar]
- 135.Liu Y., Sonek G.J., Berns M.W., Tromberg B.J. Physiological monitoring of optically trapped cells: assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry. Biophys. J. 1996;71:2158–2167. doi: 10.1016/S0006-3495(96)79417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liang H., Vu K.T., Krishnan P., Trang T.C., Shin D. Wavelength dependence of cell cloning efficiency after optical trapping. Biophys. J. 1996;70:1529–1533. doi: 10.1016/S0006-3495(96)79716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neuman K.C., Chadd E.H., Liou G.F., Bergman K., Block S.M. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 1999;77:2856–2863. doi: 10.1016/S0006-3495(99)77117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mohanty S.K., Rapp A., Monajembashi S., Gupta P.K., Greulich K.O. Comet assay measurements of DNA damage in cells by laser microbeams and trapping beams with wavelengths spanning a range of 308 nm to 1064 nm. Rad. Res. 2002;157:378–385. doi: 10.1667/0033-7587(2002)157[0378:camodd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 139.Ayano S., Wakamoto Y., Yamashita S., Yasuda K. Quantitative measurement of damage caused by 1064-nm wavelength optical trapping of Escherichia coli cells using on-chip single cell cultivation system. Biochem. Biophys. Res. Commun. 2006;350:678–684. doi: 10.1016/j.bbrc.2006.09.115. [DOI] [PubMed] [Google Scholar]
- 140.Mohanty S.K., Sharma M., Gupta P.K. Generation of ROS in cells on exposure to CW and pulsed near-infrared laser tweezers. Photochem. Photobiol. Sci. 2006;5:134–139. doi: 10.1039/b506061c. [DOI] [PubMed] [Google Scholar]
- 141.Sahu K., Mohanty S.K., Gupta P.K. He–Ne laser (632.8 nm) pre-irradiation gives protection against DNA damage induced by a near-infrared trapping beam. J. Biophoton. 2009;2:140–144. doi: 10.1002/jbio.200810041. [DOI] [PubMed] [Google Scholar]
- 142.Pilát Z., Ježet J., Šerý M., Trtílek M., Nedbal L. Optical trapping of microalgae at 735–1064 nm: photodamage assessment. J. Photochem. Photobiol. B: Biol. 2013;121:27–31. doi: 10.1016/j.jphotobiol.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 143.Hawkins-Evans D., Abrahamse H. Efficacy of three different laser wavelengths for in vitro wound healing. Photodermatol. Photoimmunol. Photomed. 2008;24:199–210. doi: 10.1111/j.1600-0781.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 144.Houreld N.N., Abrahamse H. Laser light influences cellular viability and proliferation in diabetic-wounded fibroblast cells in a dose- and wavelength-dependent manner. Lasers Med. Sci. 2008;23:11–18. doi: 10.1007/s10103-007-0445-y. [DOI] [PubMed] [Google Scholar]
- 145.Mirsaidov U., Timp W., Timp K., Mir M., Matsudaira P. Optimal optical trap for bacterial viability. Phys. Rev. E. 2008;78:021910. doi: 10.1103/PhysRevE.78.021910. [DOI] [PubMed] [Google Scholar]
- 146.Spitler R., Berns M.W. Comparison of laser and diode sources for acceleration of in vitro wound healing by low-level light therapy. J. Biomed. Opt. 2014;19:038001. doi: 10.1117/1.JBO.19.3.038001. [DOI] [PubMed] [Google Scholar]
- 147.Leitz G., Fällman E., Tuck S., Axner O. Stress response in Caenorhabditis elegans caused by optical tweezers: wavelength, power, and time dependence. Biophys. J. 2002;82:2224–2231. doi: 10.1016/S0006-3495(02)75568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Murayama H., Sadakane K., Yamanoha B., Kogure S. Low-power 808-nm laser irradiation inhibits cell proliferation of a human-derived glioblastoma cell line in vitro. Lasers Med. Sci. 2012;27:87–93. doi: 10.1007/s10103-011-0924-z. [DOI] [PubMed] [Google Scholar]
- 149.da Silva Sergio L.P., Almeida da Silva A.P., Amorim P.F., Araújo Campos V.M., Magalhães L.A. Gonçalves. DNA damage in blood cells exposed to low-level lasers. Lasers Surg. Med. 2015;47:361–368. doi: 10.1002/lsm.22344. [DOI] [PubMed] [Google Scholar]
- 150.Trajano L.A.S.N., Sergio L.P.S., Silva C.L., Carvalho L., Mencalha A.L. Low-level laser irradiation alters mRNA expression from genes involved in DNA repair and genomic stabilization in myoblasts. Laser Phys. Lett. 2016;13:075601. [Google Scholar]
- 151.da Silva Neto Trajano L.A., da Silva C.L., Nunes de Carvalho S., Cortez E., Mencalha A.L. Cell viability, reactive oxygen species, apoptosis, and necrosis in myoblast cultures exposed to low-level infrared laser. Lasers Med. Sci. 2016;31:841–848. doi: 10.1007/s10103-016-1909-8. [DOI] [PubMed] [Google Scholar]
- 152.Almeida L.G., Sergio L.P.S., Vicentini S.C., Mencalha A.L., Paoli F. Nuclear phenotype evaluation in skeletal muscle from Wistar rats exposed to low-level lasers. Laser Phys. 2017;27:035601. [Google Scholar]
- 153.Yu Z., Li Z., Liu N., Jizhang Y., McCarthy T.J. Near infrared radiation protects against oxygen-glucose deprivation-induced neurotoxicity by down-regulating neuronal nitric oxide synthase (nNOS) activity in vitro. Metab. Brain Dis. 2015;30:829–837. doi: 10.1007/s11011-015-9663-3. [DOI] [PubMed] [Google Scholar]
- 154.Yu Z., Liu N., Zhao J., Li Y., McCarthy T.J. Near infrared radiation rescues mitochondrial dysfunction in cortical neurons after oxygen-glucose deprivation. Metab. Brain Dis. 2015;30:491–496. doi: 10.1007/s11011-014-9515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin L.R., Cohen R.B., Schatz J.F. Quenching of laser induced fluorescence of O2 (b 1Σ+g) by O2 and N2. Chem. Phys. Lett. 1976;41:394–396. [Google Scholar]
- 156.Knickelbein M.B., Marsh K.L., Ulrich O.E., Busch G.E. Energy transfer kinetics of singlet molecular oxygen: the deactivation channel for O2(b 1Σ+g) J. Chem. Phys. 1987;87:2392–2393. [Google Scholar]
- 157.Wildt J., Bednarek G., Fink E.H. Laser excitation of O2 (b 1Σ+g, v´=0, 1, 2) – rates and channels of energy transfer and quenching. Chem. Phys. 1988;122:463–470. [Google Scholar]
- 158.Xu S., Dai D., Xie J., Sha G., Zhang C. Quantitative measurements of O2 b ← X(2,1,0←0) bands by using cavity ring-down spectroscopy. Chem. Phys. Lett. 1999;303:171–175. [Google Scholar]
- 159.Cheah S.L., Lee Y.P., Ogilvie J.F. Wavenumbers, strengths, widths and shifts with pressure of lines in four bands of gaseous 16O2 in the systems a1Δg - X3Σ-g and b1Σ+g – X3Σ-g. J. Quant. Spectrosc. Rad. Trans. 2000;64:467–482. [Google Scholar]
- 160.Pohlkötter A., Köhring M., Willer U., Schade W. Detection of `opy. Sensors. 2010;10:8466–8477. doi: 10.3390/s100908466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vorobjev I.A., Liang H., Wright W.H., Berns M.W. Optical trapping for chromosome manipulation: a wavelength dependence of induced chromosome bridges. Biophys. J. 1993;64:533–538. doi: 10.1016/S0006-3495(93)81398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.König K., Liang H., Berns M.W., Tromberg B.J. Cell damage by near-IR microbeams. Nature. 1995;377:20–21. doi: 10.1038/377020a0. [DOI] [PubMed] [Google Scholar]
- 163.König K., Liang H., Berns M.W., Tromberg B.J. Cell damage in near-infrared multimode optical traps as a result of multiphoton absorption. Opt. Lett. 1996;21:1090–1092. doi: 10.1364/ol.21.001090. [DOI] [PubMed] [Google Scholar]
- 164.Liang H., Vu K.T., Trang T.C., Shin D., Lee Y.E. Giant cell formation in cells exposed to 740 nm and 760 nm optical traps. Lasers Surg. Med. 1997;21:159–165. doi: 10.1002/(sici)1096-9101(1997)21:2<159::aid-lsm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 165.König K., So P.T.C., Mantulin W.W., Gratton E. Cellular response to near-infrared femtosecond laser pulses in two-photon microscopes. Opt. Lett. 1997;22:135–136. doi: 10.1364/ol.22.000135. [DOI] [PubMed] [Google Scholar]
- 166.Lawton S.A., Novick S.E., Broida H.P., Phelps A.V. Quenching of optically pumped O2(b1Σ+g) by ground state O2 molecules. J. Chem. Phys. 1977;66:1381–1382. [Google Scholar]
- 167.Bloemink H.I., Copeland R.A., Slanger T.G. Collisional removal of O2 (b 1Σ+g, v=1, 2) by O2, N2, and CO2. J. Chem. Phys. 1998;109:4237–4245. [Google Scholar]
- 168.Almeida-Lopes L., Rigau J., Zângaro R.A., Guidugli-Neto J.J., Martins Marques Jaeger M. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg. Med. 2001;29:179–184. doi: 10.1002/lsm.1107. [DOI] [PubMed] [Google Scholar]
- 169.Karu T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. (DOI: 10.1111⁄j.1751-1097.2008.00394.x) [DOI] [PubMed] [Google Scholar]
- 170.Passarella S., Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B: Biol. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 171.Ryter S.W., Tyrrell R.M. Singlet molecular oxygen (1O2): a possible effector of eukaryotic gene expression. Free Rad. Biol. Med. 1998;24:1520–1534. doi: 10.1016/s0891-5849(97)00461-9. [DOI] [PubMed] [Google Scholar]
- 172.Tyrrell R.M. Modulation of gene expression by the oxidative stress generated in human skin cells by UVA radiation and the restoration of redox homeostasis. Photochem. Photobiol. Sci. 2012;11:135–147. doi: 10.1039/c1pp05222e. [DOI] [PubMed] [Google Scholar]
- 173.Sil´dos I.R., Rebane L.A., Treshchalov A.B., Lykhmus A.É. Luminescence of pairs of laser-excited oxygen molecules. JETP Lett. 1975;22:151–152. [Google Scholar]
- 174.Koroll G.W., Singh A. Use of a high intensity dye laser to produce singlet oxygen. Photochem. Photobiol. 1978;28:611–613. [Google Scholar]
- 175.Furui E., Akai N., Ida A., Kawai A., Shibuya K. Observation of collision-induced near-IR emission of singlet oxygen O2 a1Δg generated by visible light excitation of gaseous O2 dimol. Chem. Phys. Lett. 2009;471:45–49. [Google Scholar]
- 176.Ida A., Furui E., Akai N., Kawai A., Shibuya K. Kinetic study on the photoabsorption process of gaseous O2 dimol at 630 nm in a wide pressure range. Chem. Phys. Lett. 2010;488:130–134. [Google Scholar]
- 177.Dube A., Bock C., Bauer E., Kohli R., Gupta P.K. He-Ne laser irradiation protects B-lymphoblasts from UVA-induced DNA damage. Radiat. Environ. Biophys. 2001;40:77–82. doi: 10.1007/s004110000086. [DOI] [PubMed] [Google Scholar]
- 178.Hu W.P., Wang J.J., Yu C.L., Lan C.C.E., Chen G.S. Helium–neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J. Invest. Dermatol. 2007;127:2048–2057. doi: 10.1038/sj.jid.5700826. [DOI] [PubMed] [Google Scholar]
- 179.Kushibiki T., Hirasawa T., Okawa S., Ishihara M. Regulation of miRNA expression by Low-Level Laser Therapy (LLLT) and Photodynamic Therapy (PDT) Int. J. Mol. Sci. 2013;14:13542–13558. doi: 10.3390/ijms140713542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ivanov A.V., Mashalov A.A., Zakharov S.D. Radioprotective action of low-intensity light into the red absorption band of endogenous molecular oxygen. J. Phys.: Conf. Ser. 2016;740:012014. [Google Scholar]
- 181.Wu S., Zhou F., Wei Y., Chen W.R., Chen Q. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid. Redox Signal. 2014;20:733–746. doi: 10.1089/ars.2013.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chung H., Dai T., Sharma S.K., Huang Y.Y., Carroll J.D. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sneep M., Ityaksov D., Aben I., Linnartz H., Ubachs W. Temperature-dependent cross sections of O2–O2 collision-induced absorption resonances at 477 and 577 nm. J. Quant. Spectrosc. Rad. Trans. 2006;98:405–424. [Google Scholar]
- 184.Van Gemert M.J.C., Welch A.J., Pickering J.W., Tan O.T., Gijsbers G.H.M. Wavelengths for laser treatment of port wine stains and telangiectasia. Lasers Surg. Med. 1995;16:147–155. doi: 10.1002/lsm.1900160204. [DOI] [PubMed] [Google Scholar]
- 185.Armichev A.V., Ivanov A.V., Panasenko N.A., Perov S.N., Zakharov S.D. Spectral dependence of erythrocyte response to low-intensity irradiation at 570–590 nm. J. Rus. Laser Res. 1995;16:186–187. [Google Scholar]
- 186.Naus H., Ubachs W. The b1Σ+g – X3Σ-g (3, 0) band of 16O2 and 18O2. J. Mol. Spectrosc. 1999;193:442–445. doi: 10.1006/jmsp.1998.7766. [DOI] [PubMed] [Google Scholar]
- 187.Kalogerakis K.S., Copeland R.A., Slanger T.G. Collisional removal of O2 (b 1Σ+g, v= 2,3) J. Chem. Phys. 2002;116:4877–4885. doi: 10.1063/1.2110227. [DOI] [PubMed] [Google Scholar]
- 188.Vogel A., Noack J., Hüttman G., Paltauf G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B. 2005;81:1015–1047. [Google Scholar]
- 189.Zakharov S.D., Korochkin I.M., Yusupov A.S., Bezotosnyi V.V., Cheshev E.A. Application of diode lasers in light-oxygen cancer therapy. Semiconductors. 2014;48:123–128. [Google Scholar]
- 190.Wai-Ying Kam W., Banati R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013;65:607–619. doi: 10.1016/j.freeradbiomed.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 191.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Graham N.A., Tahmasian M., Kohli B., Komisopoulou E., Zhu M. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol. Syst. Biol. 2012;8:589. doi: 10.1038/msb.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhou X., Wang Y., Si J., Zhou R., Gan L. Laser controlled singlet oxygen generation in mitochondria to promote mitochondrial DNA replication in vitro. Sci. Rep. 2015;5:16925. doi: 10.1038/srep16925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou X., Wang Y., Si J., Zhou R., Gan L. Laser controlled singlet oxygen generation in mitochondria to promote mitochondrial DNA replication in vitro. Sci. Rep. 2015;5:16925. doi: 10.1038/srep16925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Prasad A., Ferretti U., Sedlářova M., Pospíšil P. Singlet oxygen production in Chlamydomonas reinhardtii under heat stress. Sci. Rep. 2016;6:20094. doi: 10.1038/srep20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rubio N., Fleury S.P., Redmond R.W. Spatial and temporal dynamics of in vitro photodynamic cell killing: extracellular hydrogen peroxide mediates neighbouring cell death. Photochem. Photobiol. Sci. 2009;8:457–464. doi: 10.1039/b815343d. [DOI] [PubMed] [Google Scholar]
- 197.Chakraborty A., Held K.D., Prise K.M., Liber H.L., Redmond R.W. Bystander effects induced by diffusing mediators after photodynamic stress. Rad. Res. 2009;172:74–81. doi: 10.1667/RR1669.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Blair I.A. Lipid hydroperoxides-mediated DNA damage. Exp. Geront. 2001;36:1473–1481. doi: 10.1016/s0531-5565(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 199.Zarkovic N., Ilic Z., Jurin M., Schaur R.J., Puhl H. Stimulation of HeLa cell growth by physiological concentrations of 4-hydroxynonenal. Cell Biochem. Func. 1993;11:279–286. doi: 10.1002/cbf.290110409. [DOI] [PubMed] [Google Scholar]
- 200.Dwivedi S., Sharma A., Patrick B., Sharma R., Awasthi Y.C. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Report. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- 201.Dubinina E.E., Dadali V.A. Role of 4-hydroxy-trans-2-nonenal in cell functions. Biochem. (Mosc.) 2010;75:1069–1087. doi: 10.1134/s0006297910090014. [DOI] [PubMed] [Google Scholar]
- 202.Ramana K.V., Bhatnagar A., Srivastava S., Yadav U.C., Awasthi S. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE) J. Biol. Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 203.Dittmann K., Mayer C., Kehlbach R., Rothmund M.C., Rodemann H.P. Radiation-induced lipid peroxidation activates src kinase and triggers nuclear EGFR transport. Radiother. Oncol. 2009;92:379–382. doi: 10.1016/j.radonc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 204.Zhang H., Forman H.J. 4-Hydroxynonenal activates Src through a non-canonical pathway that involves EGFR/PTP1B. Free Rad. Biol. Med. 2015;89:701–707. doi: 10.1016/j.freeradbiomed.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Awasthi Y.C., Yang Y., Tiwari N.K., Patrick B., Sharma A. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Rad. Biol. Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 206.Li W.G., Stoll L.L., Rice J.B., Xu S.P., Miller F.J. Activation of NAD(P)H oxidase by lipid hydroperoxides: mechanism of oxidant-mediated smooth muscle cytotoxicity. Free Rad. Biol. Med. 2003;34:937–946. doi: 10.1016/s0891-5849(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 207.Mallikarjun V., Clarke D.J., Campbell C.J. Cellular redox potential and the biomolecular electrochemical series: a systems hypothesis. Free Rad. Biol. Med. 2012;53:280–288. doi: 10.1016/j.freeradbiomed.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 208.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signaling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 209.Blázquez-Castro A., Stockert J.C. In vitro human cell responses to a low-dose photodynamic treatment vs. mild H2O2 exposure. J. Photochem. Photobiol. B: Biol. 2015;143:12–19. doi: 10.1016/j.jphotobiol.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 210.Jones D.P. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J. Intern. Med. 2010;268:432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gollmer A., Besostri F., Breitenbach T., Ogilby P.R. Spatially resolved two-photon irradiation of an intracellular singlet oxygen photosensitizer: correlating cell response to the site of localized irradiation. Free Rad. Res. 2013;47:718–730. doi: 10.3109/10715762.2013.817670. [DOI] [PubMed] [Google Scholar]
- 212.Perakis F., De Marco L., Shalit A., Tang F., Kann Z.R. Vibrational spectroscopy and dynamics of water. Chem. Rev. 2016;116:7590–7607. doi: 10.1021/acs.chemrev.5b00640. [DOI] [PubMed] [Google Scholar]
- 213.Bagrov I.V., Belousova I.M., Kiselev V.M., Kislyakov I.M., Sosnov E.N. Observation of the Luminescence of Singlet Oxygen at λ = 1270 nm under LED Irradiation of CCl4. Opt. Spectrosc. 2012;113:57–62. [Google Scholar]
- 214.Bagrov I.V., Kiselev V.M., Kislyakov I.M., Sosnov E.N. Direct optical excitation of singlet oxygen in organic solvents. Opt. Spectrosc. 2014;116:567–574. [Google Scholar]
- 215.Kiselev V.M., Kislyakov I.M., Bagrov I.V. Spectral dependence of the efficiency of direct optical excitation of molecular oxygen in tetrachloromethane. Opt. Spectrosc. 2016;120:859–863. [Google Scholar]
- 216.Zipfel W.R., Williams R.M., Webb W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotech. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 217.Hoover E.E., Squier J.A. Advances in multiphoton microscopy technology. Nat. Phot. 2013;7:93–101. doi: 10.1038/nphoton.2012.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.S.D. Zakharov, N.C. Thanh, Photogenerated Singlet Oxygen Damages Cells in Optical Traps, 2009arXiv0911.4651T, 2009.
- 219.Kuimova M.K., Botchway S.W., Parker A.W., Balaz M., Collins H.A. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009;1:69–73. doi: 10.1038/nchem.120. [DOI] [PubMed] [Google Scholar]
- 220.Liu Y., Cheng D.K., Sonek G.J., Berns M.W., Chapman C.F. Evidence for localized cell heating induced by infrared optical tweezers. Biophys. J. 1995;68:2137–2144. doi: 10.1016/S0006-3495(95)80396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schönle A., Hell S.W. Heating by absorption in the focus of an objective lens. Opt. Lett. 1998;23:325–327. doi: 10.1364/ol.23.000325. [DOI] [PubMed] [Google Scholar]
- 222.Peterman E.J.G., Gittes F., Schmidt C.F. Laser-Induced Heating in Optical Traps. Biophys. J. 2003;84:1308–1316. doi: 10.1016/S0006-3495(03)74946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dressler C., Beuthan J., Mueller G., Zabarylo U., Minet O. Fluorescence imaging of heat-stress induced mitochondrial long-term depolarization in breast cancer cells. J. Fluoresc. 2006;16:689–695. doi: 10.1007/s10895-006-0110-z. [DOI] [PubMed] [Google Scholar]
- 224.Zukiene R., Nauciene Z., Ciapaite J., Mildažienė V. Acute temperature resistance threshold in heart mitochondria: febrile temperature activates function but exceeding it collapses the membrane barrier. Int. J. Hyperth. 2010;26:56–66. doi: 10.3109/02656730903262140. [DOI] [PubMed] [Google Scholar]
- 225.White M.G., Saleh O., Nonner D., Barrett E.F., Moraes C.T. Mitochondrial dysfunction induced by heat stress in cultured rat CNS neurons. J. Neurophysiol. 2012;108:2203–2214. doi: 10.1152/jn.00638.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Crisan B., Soritau O., Baciut M., Campian R., Crisan L. Influence of different lasers wavelengths on nanoparticles components of human fibroblasts. Part. Sci. Tech. 2013;31:168–173. doi: 10.1007/s10103-012-1084-5. [DOI] [PubMed] [Google Scholar]
- 227.Wang Z., Cai F., Chen X., Luo M., Hu L. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS One. 2013;8:e75044. doi: 10.1371/journal.pone.0075044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Slimen I.B., Najar T., Ghram A., Dabbebi H., Mrad M.B. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014;30:513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 229.Kikusato M., Yoshida H., Furukawa K., Toyomizu M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and hemeoxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 2015;52:8–13. doi: 10.1016/j.jtherbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 230.Khan I., Tang E., Arany P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci. Rep. 2015;5:10581. doi: 10.1038/srep10581. [DOI] [PMC free article] [PubMed] [Google Scholar]