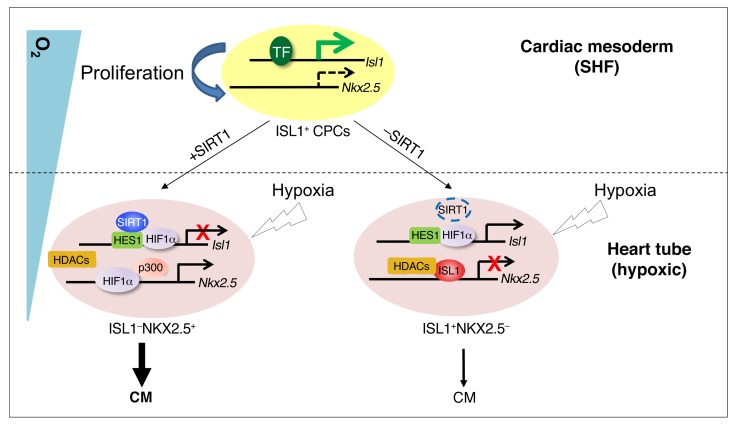
Figure 7. Schematic model of the regulation of ISL1+ CPC behavior by hypoxia, transcription factors, and epigenetic modifiers during early heart development.
ISL1+ CPCs maintain high Isl1 expression directed by transcription activators such as β-catenin/LEF, FOXO1, and GATA4 in response to inductive signals, but low Nkx2.5 expression levels in the cardiac mesoderm, where O2 concentrations are relatively high, favoring self-renewal and expansion. ISL1 recruits HDACs to the Nkx2.5 promoter in CPCs to repress Nkx2.5 expression and prevent precocious myocyte specification. Migration of ISL1+ cells into the growing physiologically hypoxic (O2 ≤2%) heart tube results in HIF1α-SIRT1-HES1–dependent silencing of Isl1 expression and HIF1α-p300–dependent stimulation of Nkx2.5 expression, promoting cardiomyocyte specification. In the absence of SIRT1, repression of Isl1 transcription is relieved, resulting in increased Isl1 levels and enhanced proliferation of CPCs. As a consequence, ISL1-mediated Nkx2.5 repression is augmented and the transition of CPCs from an ISL1+NKX2.5– to an ISL1–NKX2.5+ state is hindered, compromising cardiomyocyte specification. TF, transcription factors.