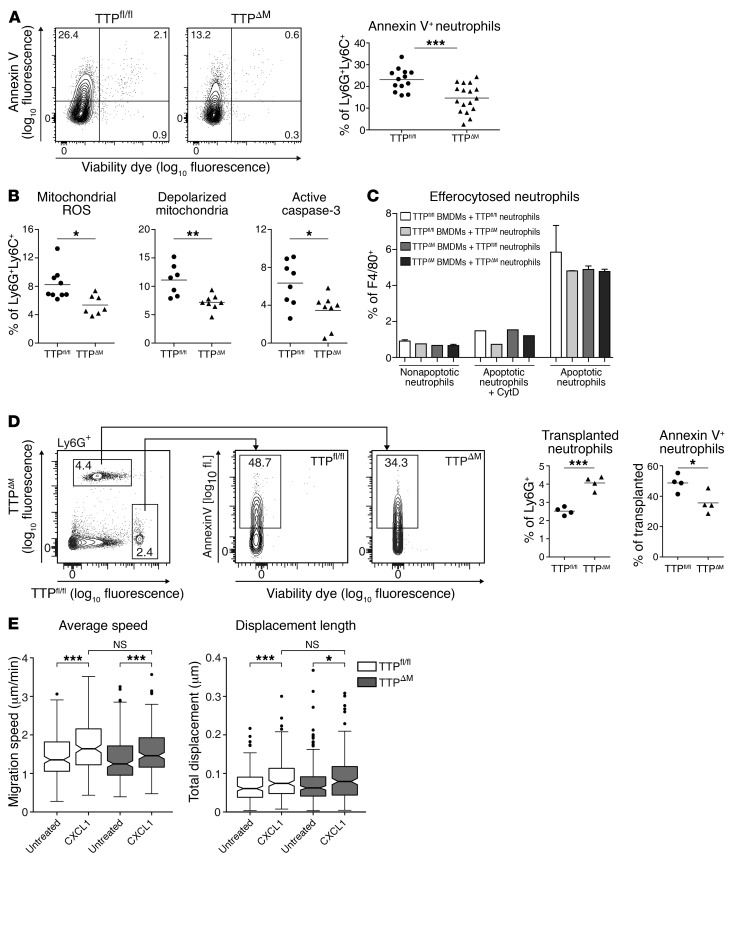
Figure 5. TTP promotes neutrophil apoptosis in a cell-intrinsic manner.
(A) Apoptosis was analyzed in peritoneal neutrophils elicited using 2 × 108 CFU HK S. pyogenes 16 hours prior to flushing of the peritoneal cavity. Representative flow plots show annexin V and live/dead staining of Ly6G+Ly6C+ neutrophils. The threshold for annexin V+ signal was based on fluorescence minus one (FMO) staining without annexin V. Numbers represent the percentage of cells in the respective quadrants. Dot plot for 3 pooled experiments (n = 13 TTPfl/fl; n = 17 TTPΔM) depicts the percentages of early apoptotic neutrophils (living annexin V+). (B) Neutrophils, elicited as in A, were analyzed for mitochondrial superoxide production (n = 9 TTPfl/fl; n = 8 TTPΔM), polarization of mitochondrial membrane (n = 7 TTPfl/fl; n = 8 TTPΔM), and active caspase-3 (n = 8 TTPfl/fl; n = 8 TTPΔM). (C) TTPfl/fl and TTPΔM BMDMs were incubated with pHrodo-stained nonapoptotic or apoptotic TTPfl/fl and TTPΔM neutrophils at a BMDM/neutrophil ratio of 1:2. CytD-pretreated BMDMs served as a control for adherent neutrophils. Bar graph shows the percentage of pHrodo+F4/80+ BMDMs (n = 3). Error bars indicate the mean ± SD. (D) Casein-elicited neutrophils from TTPfl/fl and TTPΔM mice were differently labeled, mixed at a 1:1 ratio, and injected i.p. into 4 recipient TTPfl/fl animals, in which peritonitis was induced 3 hours earlier using HK S. pyogenes. Representative flow plots 18 hours after transplantation and dot plots show retrieved transplanted neutrophils and the percentage of annexin V+ cells therein. (E) Neutrophil migration through a collagen matrix was followed by live imaging. Box plots show the average speed and displacement length of TTPfl/fl and TTPΔM neutrophils in untreated conditions or in a CXCL1 gradient. Error bars in A, B, and D represent the mean. Statistical analysis was determined by unpaired (A–D) or paired (E) Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001.