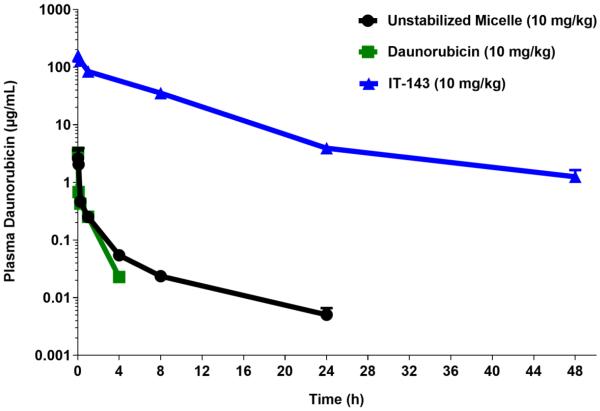
Figure 3.
Daunorubicin-loaded, iron-stabilized micelle formulation (IT-143) demonstrates prolonged circulation compared to unstabilized micelle formulation and free drug in a cannulated rat model. Intravenous administration of IT-143 resulted in exposure to the plasma compartment (AUC0-48h) of 913.7 µg*h/mL compared to 1.5 and 0.96 µg*h/mL for unstabilized micelle formulation and daunorubicin free drug, respectively.