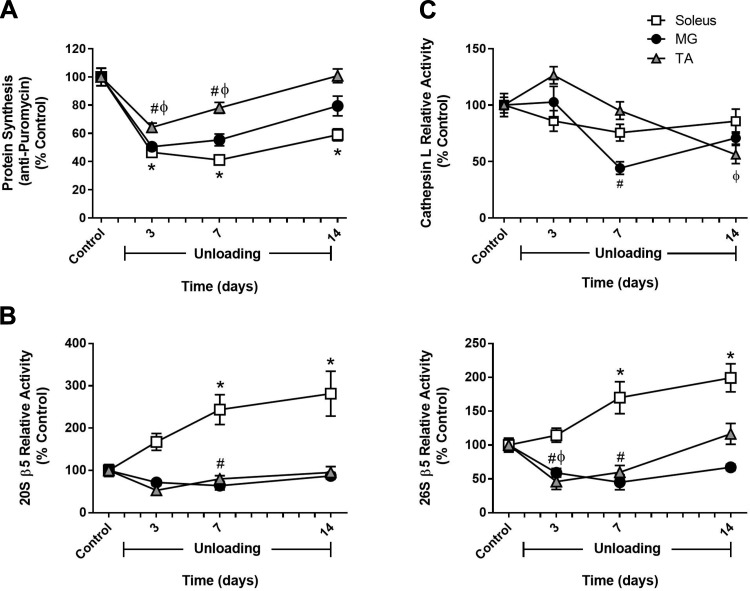
Fig. 2.
Comparison of changes in muscle protein synthesis and protein degradation between hindlimb muscles in response to unloading. Adult (9 mo) male rats underwent hindlimb unloading (HU) for 3, 7, or 14 days. Protein synthesis (A), 20S and 26S β5 proteasome subunit activity (B), and cathepsin L activity (C) were measured in the soleus (open squares), medial gastrocnemius (MG; solid circles), and tibialis anterior (TA; shaded triangles) at each unloading time point. Total protein determined by stain-free imaging of the PVDF membrane was used to normalize puromycin protein expression. All values are expressed as a percentage relative to the control group for each muscle (n = 5–6/muscle). Values are means ± SE. *P < 0.05 vs. control soleus. #P < 0.05 vs. control MG. ϕP < 0.05 vs. control TA.