Resolution of the dynamic stimulus-response profile provides a greater understanding of the underlying physiological control processes than steady-state measurements alone. We report a novel method of measuring cerebrovascular blood velocity (MCAv) kinetics under ecologically valid conditions from rest to moderate-intensity exercise. This technique reveals that brain blood flow increases exponentially following the onset of exercise with 1) a strong bilateral coherence in young healthy individuals, and 2) a potential for unique age- and disease-specific profiles.
Keywords: brain blood flow, middle cerebral artery, blood flow velocity, aging, stroke
Abstract
The dynamic response to a stimulus such as exercise can reveal valuable insights into systems control in health and disease that are not evident from the steady-state perturbation. However, the dynamic response profile and kinetics of cerebrovascular function have not been determined to date. We tested the hypotheses that bilateral middle cerebral artery blood flow mean velocity (MCAV) increases exponentially following the onset of moderate-intensity exercise in 10 healthy young subjects. The MCAV response profiles were well fit to a delay (TD) + exponential (time constant, τ) model with substantial agreement for baseline [left (L): 69, right (R): 64 cm/s, coefficient of variation (CV) 11%], response amplitude (L: 16, R: 13 cm/s, CV 23%), TD (L: 54, R: 52 s, CV 9%), τ (L: 30, R: 30 s, CV 22%), and mean response time (MRT) (L: 83, R: 82 s, CV 8%) between left and right MCAV as supported by the high correlations (e.g., MRT r = 0.82, P < 0.05) and low CVs. Test-retest reliability was high with CVs for the baseline, amplitude, and MRT of 3, 14, and 12%, respectively. These responses contrasted markedly with those of three healthy older subjects in whom the MCAV baseline and exercise response amplitude were far lower and the kinetics slowed. A single older stroke patient showed baseline ipsilateral MCAV that was lower still and devoid of any exercise response whatsoever. We conclude that kinetics analysis of MCAV during exercise has significant potential to unveil novel aspects of cerebrovascular function in health and disease.
NEW & NOTEWORTHY Resolution of the dynamic stimulus-response profile provides a greater understanding of the underlying the physiological control processes than steady-state measurements alone. We report a novel method of measuring cerebrovascular blood velocity (MCAv) kinetics under ecologically valid conditions from rest to moderate-intensity exercise. This technique reveals that brain blood flow increases exponentially following the onset of exercise with 1) a strong bilateral coherence in young healthy individuals, and 2) a potential for unique age- and disease-specific profiles.
vascular control across different organs subserves a range of primary requirements from thermoregulation in the skin to blood filtration in the kidney and support of cellular energetics in the heart, skeletal muscle, and brain. In each organ blood flow control must be regulated in accordance with the required function within an error tolerance presumably dictated by the extent of damage incurred to the organ or organism by hypoperfusion. The brain does not endure the up to 100-fold increases in oxygen demand (V̇o2) incurred by vigorously contracting muscle(s). However, it supports extremely high oxidative function at rest and this increases in response to the neuromuscular activation requirements of physical exercise (15, 25, 34, 36, 37, 43, 49, 52, 61).
Unlike skeletal muscle, the brain is notably lacking in energy storage mechanisms (28, 58, 70) and increases its fractional oxygen extraction to ~50% rather than the 80–90% found in skeletal muscle (25, 51). Thus, once thought to be constant (41), there is now substantial evidence that cerebrovascular blood flow increases during exercise (15, 20, 25, 27, 32, 34, 36, 37, 43, 52, 61). Moreover, that increase is highest, and laterally symmetrical, for cycling at moderate exercise intensities but reduced for vigorous and maximal intensities where hyperventilation and hypocapnia induce cerebral arteriolar vasoconstriction (25, 31, 43, 49, 52, 61, 69). Given the brain’s lack of O2 stores, and intolerance to anoxia, that elevated blood flow and oxygen delivery must be rapidly and precisely matched to the brain’s metabolic requirements.
Whereas quantitative steady-state responses of blood flow, ventilation, or V̇o2 can provide valuable information, resolution of the response kinetics to a change in demand from rest to exercise, for example, can uncover sentinel features of the underlying control mechanisms. Such analyses are technically and mathematically challenging but also extremely rewarding. For instance, kinetics analyses of pulmonary and muscle V̇o2 have demonstrated that, in health but not disease (e.g., heart failure, type II diabetes, and chronic obstructive pulmonary disease), the speed of the kinetics response at exercise onset is limited by mitochondrial function and not O2 delivery (5, 7, 51). At the arteriolar level, kinetics analyses of vasodilation (and vasoconstriction) have extended these findings and explain how O2 delivery temporally and quantitatively matches oxidative demands in healthy young but not aged muscles (6, 39, 40, 54). Similar analyses for ventilation have unveiled how the carotid body facilitates ventilatory dynamics and blood-gas and acid-base regulation across metabolic transitions (67). It is surprising therefore that cerebrovascular kinetics have not been studied to date.
The exercise hyperemia of the brain has been demonstrated with anterior and middle cerebral artery blood velocity (MCAV) (15, 34, 36, 37, 43) and “steady-state” cerebral blood flow increasing by 10–30% following the onset of low- and moderate-intensity exercise (21, 35, 36). It is also thought that life-long physical activity results in greater cerebral blood flow to support brain tissue, possibly through angiogenesis and improved cerebrovascular responsiveness to demand (1, 4). In this regard, cerebral endothelial nitric oxide synthase levels are increased following an exercise training program in rodents (48, 71) supporting that chronic exercise elevates cerebrovascular control as seen for the coronary and skeletal muscle vasculatures (42). It is not known whether such training improves either the speed or amplitude of the hyperemic response of brain blood flow in response to physical exercise or alternatively whether that response is altered or compromised by age and or diseases such as Alzheimer’s, stroke, diabetes, or heart failure, in part, because kinetics analyses of cerebrovascular blood flow have not been conducted.
The MCA was chosen as the vessel of interest for assessing the kinetics of cerebrovascular regulation since previous work has focused on the MCA during exercise (1, 21, 35, 36, 55, 62). Here we report the use of a novel analysis method for assessing the kinetics of cerebrovascular regulation to provide the first characterization of the dynamic cerebrovascular [specifically, the middle cerebral artery blood mean velocity, mean MCAV (termed MCAv throughout)] response to exercise. Specifically, we tested the hypotheses that 1) following the onset of exercise MCAV increases exponentially; 2) the parameters of this response (amplitude, time constant, and mean response time) are not systematically different between right and left MCA, and 3) the kinetics profile and kinetics parameters may be substantially altered by age and disease (stroke). Specifically, we demonstrate the putative application of this technique for characterizing differences across age and disease states with the future purpose of identifying and testing therapeutic strategies for better preserving, or recovering, cerebrovascular and, potentially, brain function.
MATERIALS AND METHODS
Ten healthy young adults with low cardiac risk (50) and three older adults without cardiovascular disease but considered high cardiac risk, in part, because of their age (50) were recruited to participate in the study. One additional individual, 88 days postischemic stroke, was recruited as a case example of known cerebrovascular injury. No individuals were considered to be competitive athletes (Table 1 contains participant demographics). The Kansas State University (KU) Medical Center Human Subjects Committee approved all experimental procedures, which complied with the Declaration of Helsinki. Institutionally approved written informed consent was obtained from each individual before participation in the study. We did not directly assess hormone level, but premenopausal females exercised around the early follicular phase of the menstrual cycle (21, 60). Female participants over 65 were considered postmenopausal.
Table 1.
Participant demographics
| Subject | Age, yr | Sex | CV Risk | Estimated V̇o2max, ml·kg−1·min−1 |
|---|---|---|---|---|
| Young | ||||
| 1 | 23 | F | L | 40.9 |
| 2 | 23 | F | L | 36.8 |
| 4 | 25 | M | L | 53.3 |
| 6 | 24 | M | L | 45.9 |
| 7 | 24 | F | L | 46.4 |
| 9 | 25 | F | L | 42.8 |
| 10 | 23 | F | L | 37.9 |
| 11 | 24 | F | L | 34.3 |
| Older | ||||
| 15 | 67 | F | H | 18.3 |
| 18 | 65 | M | H | 28.0 |
| 20 | 66 | F | H | 18.9 |
| Stroke patient | ||||
| 201 | 65 | M | H | * |
CV, cardiovascular; L, low; H, high; F, female; M, male.
The stroke participant had a low resting heart rate secondary to β-blocker use, negating estimation of V̇o2max.
Participants were screened either over the phone or in person. Inclusion criteria were 1) 20–85 yr of age, 2) ability to perform repeated bouts of moderate-intensity exercise, and 3) transportation to KU Medical Center for testing. Exclusion criteria were 1) inability of study staff to acquire a signal of the MCA using transcranial Doppler ultrasound; 2) inability to perform the alternating leg movements on the seated recumbent stepper (T5XR NuStep, Ann Arbor, MI); 3) diagnosis of Parkinson’s disease, mild cognitive impairment, Alzheimer’s disease, or multiple sclerosis; and 4) pulmonary disease or dependency on supplemental oxygen. Before reporting to the laboratory at KU Medical Center, participants were asked to abstain from the following before testing: food for 2 h before testing (21), caffeine for at least 6 h, and vigorous exercise for 12 h. The laboratory room for the experimental session was dimly lit, quiet and temperature maintained between 22 and 24°C (11, 12). External stimuli were kept to a minimum.
After written informed consent was obtained, resting heart rate (HR) was taken by a handheld device (Tuffsat Ohmeda; GE Healthcare, Chicago, IL). Then, the participant was familiarized with the equipment and procedures. All participants were instructed to breathe only through their nose during the experiments. A nasal cannula was placed in the participants’ nares, and, if needed, adjustments to the position of the nasal cannula were made to ensure optimal end-tidal carbon dioxide ( in mmHg) reading (BCI Capnocheck Sleep 9004; Smiths Medical, Dublin, OH). The nasal cannula remained in place at rest and during exercise familiarization to allow the individual to practice breathing through their nose. During testing, participants were observed closely to ensure breathing was exclusively through their nose.
Participants practiced the reciprocal motion of the recumbent stepper at the prescribed rate of 120 steps/min. The recumbent stepper was used for this study since it is the modality of choice for older adults (44) and is often used with those after stroke due to motor and balance impairments (8–10, 13). Next, a target work rate was identified by setting the resistance to 40 W and then increasing at a rate of 10 W every 30 s until their target HR for moderate-intensity exercise was achieved and maintained for 1 min. Moderate-intensity exercise was defined as 45–55% of HR reserve calculated using the Karvonen formula and age-predicted maximum heart rate of 220 age (50). Work rates estimated to be in the upper region of the moderate-intensity domain were specifically selected because we wished to evoke the greatest increase in MCAV (25, 31, 43, 49, 52, 61, 69). The lactic acidosis and consequent hyperventilation associated with vigorous and maximal intensity exercise were avoided because hypocapnia induces cerebral vasoconstriction and reduced cerebral blood flow (69). After the target work rate was found, study staff then completed the American College of Sports Medicine cardiac risk stratification (50), nonexercise V̇o2max estimate questionnaire (38), participant demographics, and information pertaining to past medical history and physical activity participation (~20 min) allowing the HR to return to within five beats of resting.
Three laboratory members conducted the experimental sessions. One individual read instructions to the participant using a standardized script, the second team member monitored beat-to-beat blood pressure (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) and , and the third team member set up and monitored the transcranial Doppler ultrasound (TCD) (Multigon Industries, Yonkers, NY), electrocardiogram (ECG) (Cardiocard; Nasiff Associates, Central Square, NY), and data acquired through an analog-to-digital data acquisition unit (NI-USB-6212; National Instruments) and custom-written software operating in MATLAB (v2014a; The Mathworks, Natick, MA).
Experimental Protocol
Set up.
Participants were seated in the semirecumbent stepper. HR was monitored continuously using V5 on the ECG.
blood pressure.
The left arm was placed on a padded table and was adjusted to ensure the arm remained at the level of the right atrium (21) and continuously monitored to ensure minimal movement during the experiment. Beat-to-beat blood pressure was acquired from the left middle finger using a finger photoplethysmograph (Finometer PRO; Finapres Medical Systems). Right arm brachial artery blood pressure was assessed with the arm at heart level using an automated sphygmomanometer with microphone (Tango M2; Suntech, Morrisville, NC). This allowed for comparison between devices to ensure accurate blood pressure measures before data collection (21).
middle cerebral artery velocity.
Middle cerebral artery velocity (MCAV) was measured using TCD at rest and during exercise. With the use of an adjustable headband, 2-MHz probes with ultrasonic gel were placed over the cranial temporal bone window (3). The MCA was accurately identified using practice standards for probe positioning and orientation, depth selection, and flow direction (3). Depth and gain settings were adjusted to ensure optimal signal strength and then the probes were fixed in place.
Experiment.
During the initial set up, the participants sat quietly for 20 min and were reminded to keep their arms and hands relaxed, to breathe through their nose, and to face forward. The recording period started with 90 s of rest. After 60 s of rest, the participant was informed that exercise would begin shortly. At 84 s, a visual countdown was provided for the participant to begin exercise. We chose to standardize exercise initiation while minimizing the ramp-up time to target intensity. All subjects began exercising at 60% of their target work rate for the subsequent moderate-intensity exercise. We increased the watts at 10-s intervals using one-third the difference of the starting and target watts until the target power was achieved resulting in attainment of the target work rate 30 s into the transition. Subjects maintained this work rate for 6 min and then cooled down for 2 min. The participants rested quietly until the HR reached plus or minus five beats of their resting HR before subsequent exercise bouts. Subjects completed three rest-to-exercise transitions, which were temporally aligned to the start of exercise and then averaged. This procedure improves the signal-to-noise ratio and thus better reveals the underlying physiological response (68).
Test-retest reliability for the baseline (BL) and criterion kinetics measurements of response amplitude (Amp), time delay (TD), time constant (τ), and mean response time (MRT, TD + τ) were established for all eight younger participants across the three transitions.
Data acquisition.
All variables were sampled at 500 Hz. To analyze, the data were divided by R-to-R cardiac interval. For each cardiac cycle, mean finger arterial pressure calculated as area under the pressure curve (MAP in mmHg), mean left and right MCAV (cm/s), , and HR (beats/min) were calculated. Data with R-to-R intervals greater than 5 Hz or changes in peak blood flow velocity greater than 10 cm/s in a single cardiac cycle were considered not physiologically real and censored. Acquisitions with more than 15% of data points censored were discarded. MCAV, MAP, and were then interpolated to 0.5 Hz using shape-preserving, piecewise cubic interpolation.
MCAV model fitting.
Kinetics analyses were conducted for the left and right MCA during exercise using 3-s time-binned mean values over the entire exercise bout with a monoexponential model:
where MCAV(t) is the MCAV at any point in time, BL is the baseline before the onset of exercise, Amp is the peak amplitude of the response, TD is the time delay proceeding the increase in MCAV, and τ is the time constant. Mean response time (MRT) was calculated as the sum of the model derived τ and TD. The total exercising MCAV response (Tot) was calculated as the sum of BL and Amp. Time-to-63% of the steady-state response was assessed as a model-independent measure of the response. Specifically, this measurement provides a nonbiased check of the model fitting without making any assumptions regarding the temporal profile of change.
Statistical Analysis
All curve fitting and statistical analyses were performed using a commercially available software package (SigmaPlot 12.5; Systat Software, San Jose, CA). Differences in resting values, exercise responses, and kinetic parameters were analyzed using Student’s paired t-tests. Differences between younger and older participants were analyzed using Student’s unpaired t-tests. Normality was verified via the Shapiro-Wilk normality test. Differences were considered significant when P < 0.05. The limits of agreement between left and right MCAV variables were investigated using the Bland-Altman method (14). Data are presented as means ± SE unless otherwise noted.
RESULTS
Young Healthy Participants
Pertinent cardiorespiratory variables.
Eight healthy young participants (6 female, 2 male) were included in the data analysis. Two subjects were excluded from kinetics analysis because a valid MCAV signal was not acquired from both the left and right MCA. The work rate for the exercise transitions was 104 ± 5 (range: 85–125) W. Consistent with moderate-intensity exercise (45–55% of HR reserve) from rest to exercise, HR increased from 86 ± 5 to 129 ± 6 beats/min (P < 0.01) and MAP increased 8 ± 4 mmHg (was increased from rest 36 ± 1 to exercise 42 ± 2 mmHg (P < 0.01).
The test retest reliability (n = 8) was high with coefficients of variation for the BL, Amp, and MRT of 3, 14, and 12%, respectively.
Midcerebral artery blood velocity.
overall response profile.
MCAV increased from rest to exercise in all young subjects with a mean Amp of 15.5 ± 3.1 (range: 9.0–35.7) cm/s for the left and 13.4 ± 2.1 (range: 7.4–25.6) cm/s for the right MCA (P = 0.21) (Table 2). As seen from the exemplar presented in Fig. 1, MCAV increased in a close-to-exponential pattern being well-fit by a delay + exponential function. This notion was supported by inspection of each individual response, analysis of the residuals (Fig. 1, line at bottom), and the high r2 of the fits themselves: left MCAV: 0.85 ± 0.03 (range: 0.73–0.97); right MCAV: 0.82 ± 0.04 (range: 0.6–0.95). In addition, the coefficient of variation of the actual time-to-63% of the steady-state response with the MRT calculated by the model was a mere 5 and 11% for the left (82.7 ± 4.1 vs. 83.3 ± 5.2 s; P = 0.78) and right MCA (81.9 ± 6.0 vs. 82.1 ± 6.5 s; P = 0.98), respectively, again indicating good model fit to the response. The delay was highly variable among subjects being 21.0–76.6 s for the left and 12.1–64.1 s for the right MCA (Table 2). Similarly, τ varied from 13.6–52.8 s for the left and 12.8–53.8 s for the right MCA. No MCAV parameter (i.e., BL, Amp, MRT, etc.) was significantly correlated with work rate or Δ (data not shown).
Table 2.
Individual MCAV baseline, total response, and kinetics parameters during moderate-intensity exercise
| MCA/Subject | BL, cm/s | Amp, cm/s | Tot, cm/s | τ, s | TD, s | MRT, s |
|---|---|---|---|---|---|---|
| Left MCA | ||||||
| Young | ||||||
| 1 | 76.7 | 11.0 | 87.7 | 36.8 | 21.0 | 57.8 |
| 2 | 74.0 | 35.7 | 109.7 | 52.8 | 46.3 | 99.1 |
| 4 | 65.3 | 18.4 | 83.7 | 30.7 | 61.8 | 92.5 |
| 6 | 62.8 | 14.8 | 77.6 | 30.4 | 50.8 | 81.2 |
| 7 | 58.1 | 9.5 | 67.6 | 19.3 | 76.6 | 95.9 |
| 9 | 68.6 | 11.9 | 80.5 | 20.0 | 55.8 | 75.8 |
| 10 | 64.4 | 9.0 | 73.4 | 13.6 | 56.0 | 69.6 |
| 11 | 84.7 | 13.6 | 98.3 | 34.6 | 59.6 | 94.2 |
| Means | 69.3 | 15.5 | 84.8 | 29.8 | 53.5 | 83.3 |
| SE | 3.1 | 3.1 | 4.8 | 4.4 | 5.6 | 5.2 |
| Older | ||||||
| 15 | 37.6 | 12.4 | 50.0 | 35.6 | 77.2 | 112.8 |
| 18 | 38.1 | 5.3 | 43.4 | 44.7 | 58.4 | 103.1 |
| 20 | 53.6 | 13.5 | 67.1 | 91.3 | 46.5 | 137.8 |
| Means | 43.1 | 10.4 | 53.5 | 57.2 | 60.7 | 117.9 |
| SE | 5.2 | 2.6 | 7.0 | 17.3 | 8.9 | 10.3 |
| Stroke | ||||||
| 201* | 37.9 | |||||
| Right MCA | ||||||
| Young | ||||||
| 1 | 68.6 | 13.0 | 81.6 | 44.0 | 12.1 | 56.1 |
| 2 | 62.7 | 25.6 | 88.3 | 40.0 | 55.5 | 95.5 |
| 4 | 60.6 | 15.2 | 75.8 | 28.5 | 64.1 | 92.6 |
| 6 | 52.8 | 10.0 | 62.8 | 29.0 | 52.1 | 81.1 |
| 7 | 49.0 | 7.4 | 56.4 | 14.1 | 63.3 | 77.4 |
| 9 | 76.5 | 11.1 | 87.6 | 15.7 | 56.0 | 71.7 |
| 10 | 65.4 | 8.4 | 73.8 | 12.8 | 55.0 | 67.8 |
| 11 | 79.5 | 16.7 | 96.2 | 53.8 | 60.5 | 114.3 |
| Means | 64.4 | 13.4 | 77.8 | 29.7 | 52.3 | 82.1 |
| SE | 3.7 | 2.1 | 4.7 | 5.4 | 5.9 | 6.5 |
| Older | ||||||
| 15 | 31.7 | 12.9 | 44.6 | 41.2 | 67.3 | 108.5 |
| 18 | 46.8 | 7.2 | 54.0 | 58.3 | 27 | 85.3 |
| 20 | 55.6 | 15.2 | 70.8 | 76.1 | 28.3 | 104.4 |
| Means | 44.7 | 11.8 | 56.4 | 58.5 | 40.9 | 99.4 |
| SE | 7.0 | 2.4 | 7.7 | 10.1 | 13.2 | 7.1 |
| Stroke | ||||||
| 201* |
Data are means ± SE. MCAV, middle cerebral artery velocity; BL, baseline; Amp, amplitude; Tot, total exercise MCAV response; τ, tau; TD, time delay; MRT, mean response time.
Kinetic parameters could not be modeled for the stroke patient (subject 201).
Fig. 1.
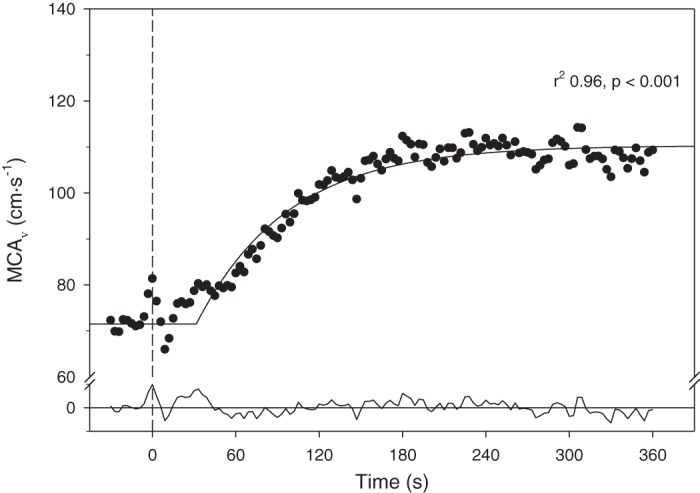
Typical middle cerebral artery velocity (MCAV) at rest and response following the onset of moderate-intensity exercise (dashed vertical line, time 0). Notice the close fit (solid curve at top) to the time delay + exponential model as supported by the high correlation coefficient and residuals profile (solid line at bottom). Results from subject 2.
Figure 2 presents the best (top) and worst (bottom) model fits from our group of young healthy subjects to emphasize that, despite some variability among subjects, the technique is tenable.
Fig. 2.
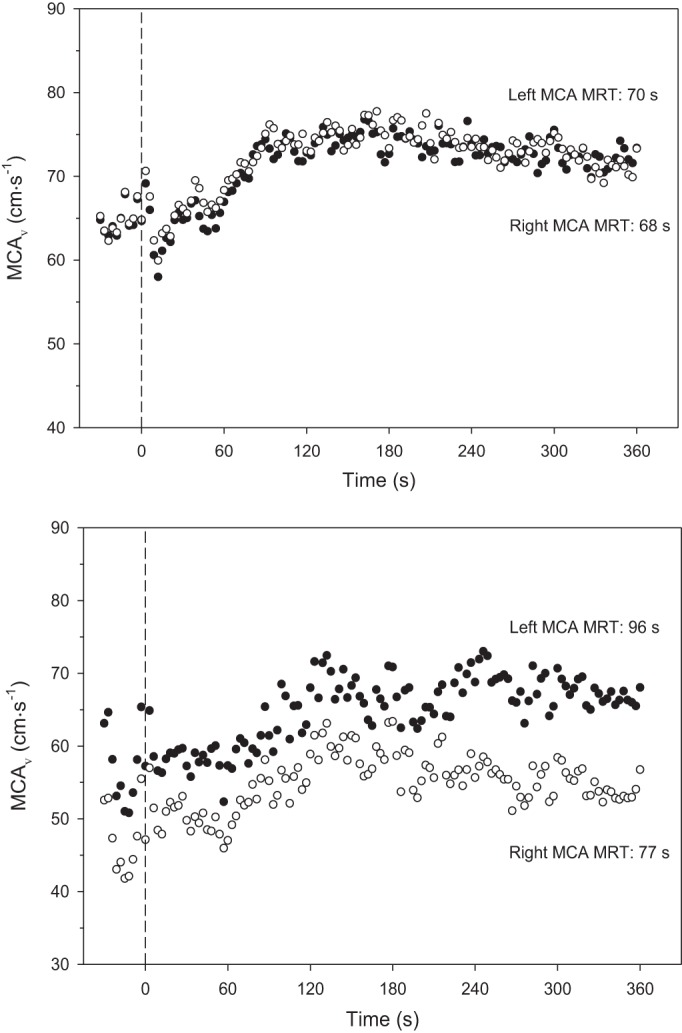
Middle cerebral artery velocity (MCAV) at rest and response following the onset of moderate-intensity exercise (dashed vertical line, time 0). Solid symbols are left MCA, and hollow symbols are right MCA. Top: illustrates subject 10 in whom there was excellent agreement between left and right. Bottom: represents poorest agreement among the young participants, subject 7. MRT, mean response time. Overall left and right MCAV correlation was 0.819 (P < 0.05) with a coefficient of variation of 7.6%.
is there systematic lateral heterogeneity?
As evident in Table 2 there was a close correspondence of the mean values between the left and right MCA for BL, Amp, TD, τ, or MRT although the coefficient of variation did differ among the parameters (i.e., 10.8% BL, 22.8% Amp, 22.1% τ, 8.7% TD, and 7.6% MRT). Figure 3 demonstrates the close correlation for the overall response (MRT, r = 0.82, P < 0.05) with this conclusion supported by the Bland-Altman plot (Fig. 3, bottom). In addition when the absolute exercising total MCAV was examined during exercise there was no difference between left (84.8 ± 4.8 cm/s) and right (77.8 ± 4.7 cm/s, P = 0.07) with a coefficient of variation of 11.1%.
Fig. 3.
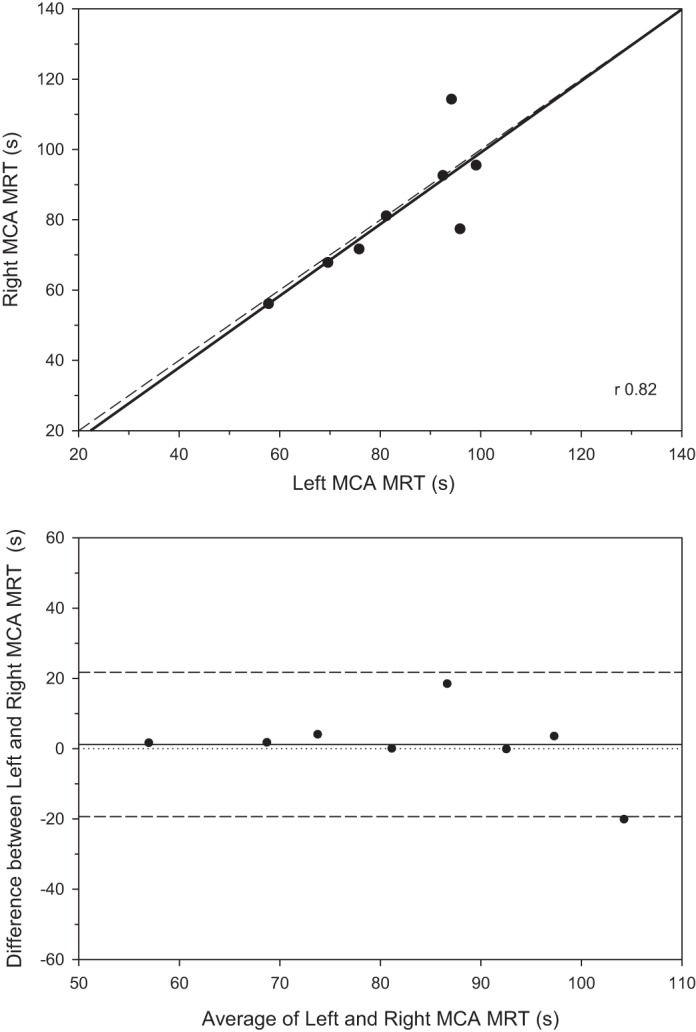
Top: correlation between left and right middle cerebral artery velocity (MCAV) mean response time (MRT) following the onset of moderate-intensity exercise (n = 8). Overall left and right MCAV correlation was 0.819 (P < 0.05) with a coefficient of variation of 7.6%. Bottom: Bland-Altman plot demonstrating the generally close correspondence between left and right MRTs. Overall left and right MCAV correlation was 0.819 (P < 0.05) with a coefficient of variation of 7.6%.
Older Adult Participants and Postischemic Stroke Patient
Older adult participants (2 female, 1 male) were considered high cardiovascular risk due to reporting existing signs/symptoms of cardiac disease [ankle edema (n = 2), orthopnea (n = 1)] and the following risk factors: age, sedentary lifestyle, hypertension, and dyslipidemia (50). The work rate for the exercise transitions was 88 ± 7 (range: 70–90) W and trended toward being significantly lower than younger participants (P = 0.06). In stark contrast to the young healthy participants, the older adults had a lower MCAV BL in both the left (43.1 ± 5.2 cm/s, P = 0.002) and right MCA (44.7 ± 7.0 cm/s, P = 0.03) as well as a markedly slowed MRT for the left (117.9 ± 10.3 s, P = 0.009) but not the right MCA (99.4 ± 7.1, P = 0.17). Despite the lower work rate, BL, and slowed kinetics for these three older subjects, the Amp was not reduced significantly in either the left (10.4 ± 2.6 cm/s, P = 0.37) or right MCA (11.8 ± 2.4 cm/s, P = 0.67) compared with the younger participants. An older subject is represented by the open circles in Fig. 4.
Fig. 4.
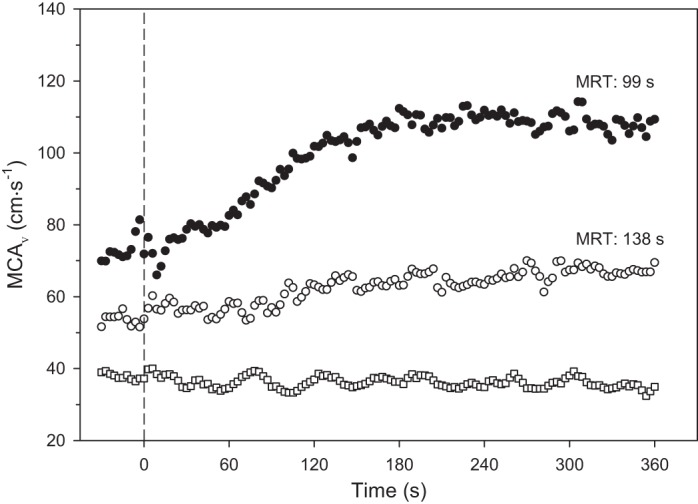
Typical middle cerebral artery velocity (MCAV) at rest and response following the onset of moderate-intensity exercise (dashed vertical line, time 0). Solid circles are from a representative young healthy subject, subject 2. Hollow circles are from an older healthy subject (subject 20). Hollow squares are from stroke patient (subject 201) using the ipsilateral MCA. By comparison, note very slow mean response time (MRT) and low amplitude of response in the older subject and absence of any response in the stroke patient.
The patient had a right ischemic stroke in the MCA territory. A dramatic response for the right MCAV was the extremely low BL (37.9 cm/s) and absence of any increase in MCAV with exercise (Fig. 4). The signal for the left MCA was not attainable.
DISCUSSION
The principal novel findings of the present investigation are aligned closely with our hypotheses. Namely, following the onset of moderate-intensity exercise, MCAV in young healthy individuals increased, after a time delay, in a close-to-exponential profile to achieve an elevated steady-state within 2–3 min. The MCAV values for BL, the response parameters (Amp, τ, MRT, TD, and Tot), and exercising steady-state were not systematically different between right and left MCA with coefficients of variation between 8 and 23% and high correlation coefficients (up to 0.82 for MRT). The three older healthy individuals exhibited lower baseline and total amplitude of MCAV response following the onset of exercise and notably slower MCAV kinetics, with τ and MRT being well outside the range of their younger healthy counterparts (i.e., 2–4 SD longer than the young average, Table 2). In marked contrast to the healthy individuals, the stroke patient exhibited a baseline that was markedly lower than the healthy young or aged subjects and displayed absolutely no increase in MCAV during exercise.
Given the current emphasis on cerebrovascular health in brain aging and disease, understanding the speed of these kinetics responses may have important clinical implications. There is mounting evidence that vascular disease, poor cerebral endothelial function, and cardiac risk factors (hypertension, hyperlipidemia) contribute to vascular dementia and Alzheimer’s disease (17, 19, 26, 53, 59, 65, 66). We may find that resolution of the kinetics response to a change in physical demand such as from rest to exercise can help clarify the interconnection between cerebrovascular health and brain function/dysfunction.
The present investigation suggests that there are not systematic differences in response kinetics or Amp between right and left MCA in healthy adults. This finding supports that either the left or right MCA can be used as an indicator of the kinetics response in both arteries during bilateral lower extremity exercise although this notion should be tested in larger and possibly more diverse populations. However, we do not know whether systematic lateral differences exist in the presence of neurologic injury such as stroke. Understanding whether true differences exist in the kinetics response between the ipsi- and contralateral vessels is an important future direction.
Experimental Considerations
Herein, we present what we hope will prove to be a sensitive technique for assessing the kinetics of cerebrovascular regulation with exercise (or other interventions). It is anticipated that determination of MCAV kinetics will be especially valuable for investigating cerebrovascular function in those at risk for, or suffering from, neurologic disease. Detecting improvements in cerebrovascular function following therapeutic or exercise interventions especially as these relate to response kinetics as well as amplitude would be a powerful capability for refining/assessing such interventions objectively. That said we recognize important limitations that must be considered.
First, we are unable to measure changes in MCA diameter. The assumption of constant MCA diameter is critical for MCAV to be used as a direct proxy for cerebral blood flow in the absence of direct diameter measures. Prior work has not permitted consensus as to whether MCA diameter changes with exercise or not (16, 29). Whereas there are some reports that MCA diameter is invariant under hypercapnic, hypocapnic, and orthostatic challenges (2, 18, 22, 56, 62–64), there is also evidence for MCA diameter changing dynamically with motor activity (23) and visual stimulation (22, 30), although fluctuations may be very modest in larger vessels such as the MCA (22, 30). It is also thought that cerebral vessels may undergo regular oscillation at rest (24). If so, a dynamic challenge such as exercise might be important for improving the signal-to-noise ratio for assessment of cerebrovascular function.
Second, the values presented in this investigation represent a small population and therefore should not be considered to span the normal range for cerebrovascular responses in young healthy adults. We provide cardiovascular risk and estimated V̇o2max as descriptive measures of general cardiovascular health. A much broader range of ages and diseases must be characterized with this technique to approach generalizability, as appropriate. Additionally, the inability to demonstrate significantly lower response Amp in the older subject cohort was undoubtedly due to the small subject number and consequently low statistical power.
Third, the kinetics parameters reported here must, to some degree, be dependent on the extant experimental conditions and not necessarily indicative of the capacity of the system to adapt to all moderate-intensity exercise challenges. For example, to minimize movement artifact we chose to increase work rate over 30 s rather than measuring the response to an immediate step increase to the intended work rate. It is likely that our kinetics parameters may have been different had we chosen a different forcing function. However, we specifically selected an exercise modality and test that does not compromise cranial stability and thus signal fidelity making them suitable for young healthy subjects as well as their older counterparts and, crucially, many stroke patients.
Fourth, changes in MAP and also the partial pressure of arterial CO2 (Pco2) can impact MCAV via alterations of driving pressure and also downstream vascular (arteriolar) resistance, respectively. For the moderate-intensity exercise used herein, MAP increased 8 mmHg (P = 0.08) during exercise. The correlation between ΔMCAV and ΔMAP was not significant with <40% of the MCAV variance being potentially explained by MAP. With respect to , it is important to note that is not the same as arterial Pco2 and the increase from 36 to 42 mmHg (P < 0.01) is expected to arise from the altered breathing pattern and rate of CO2 evolution rather than altered arterial Pco2. Indeed, for moderate-intensity exercise a substantial amount of literature supports that humans regulate their arterial Pco2 ~40 mmHg (33). As for MAP, there was no significant correlation between changes in and MCAV. For our experimental purposes, it was important to track to ensure that the subjects did not hyperventilate (i.e., drive down ) due to excitement, nervousness or some other nonspecific ventilatory responses, which would have induced cerebral arteriolar vasoconstriction. As the -arterial Pco2 difference is inversely related to breathing frequency and directly related to tidal volume and CO2 output, for the exercise intensity herein the work of Jones et al. (33) supports that an increase in of 3–5 mmHg would be expected in the absence of altered arterial CO2.
Perspectives
Current work in healthy adults considers that moderate-intensity exercise may be beneficial for motor learning (57) while other studies report high or vigorous intensity results in improved performance (46, 47). If the premise for improving motor learning is related to increased cerebral blood flow, we need to consider what exercise intensity, duration, and frequency evoke an optimal cerebral blood flow response for healthy individuals and those with brain-related pathology (45). As mentioned previously, there is evidence that moderate exercise intensities do increase cerebral blood flow whereas cerebral arteriolar vasoconstriction reduces blood flow at higher exercise intensities due to the arterial acidemia and consequent peripheral chemoreceptor-mediated hyperventilation and hypocapnia (25, 31, 43, 49, 52, 61, 69). Future work should include the MCAV kinetics response in addition to Amp and consider how they relate across specific age groups and chronic health conditions and whether there might be gender-related differences.
In conclusion, we have described a method for assessing the kinetics of cerebrovascular response to exercise. We present evidence for symmetrical vascular responses in young healthy subjects and preliminary data supporting notable differences in the MCAV response kinetics parameters related to age and cerebrovascular damage. With the growing interest in exercise benefits for the brain, ecologically valid methods for measuring cerebrovascular response kinetics across the transition from rest to exercise may become an established investigative technique. Future work should explore the reliability, sensitivity, and specificity of this technique across populations of interest with a view to determining the potential efficacy of therapeutic interventions designed to improve cerebrovascular health and neurological function.
GRANTS
S. A. Billinger was supported in part by National Institute of Child Health and Human Development (NICHD) Grant K01-HD-067318. J. F. Sisante and S. J. Kwapiszeski were supported in part by NICHD Grant T32-HD-057850. E. D. Vidoni received partial support from the University of Kansas Alzheimer's Disease Center (P30AG035982). D. C. Poole and J. C. Craig were supported, in part, through National Heart, Lung, and Blood Institute Grant HL-2-108328. The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A.B., S.J.K., E.D.V., and R.M. conceived and designed research; S.A.B., J.C.C., S.J.K., J.-F.V.S., E.D.V., R.M., and D.C.P. analyzed data; S.A.B., J.C.C., R.M., and D.C.P. interpreted results of experiments; S.A.B., J.C.C., S.J.K., E.D.V., and D.C.P. drafted manuscript; S.A.B., J.C.C., J.-F.V.S., E.D.V., and D.C.P. edited and revised manuscript; S.A.B. approved final version of manuscript; J.C.C., E.D.V., and D.C.P. prepared figures; S.J.K. and J.-F.V.S. performed experiments.
ACKNOWLEDGMENTS
We thank Anna E. Mattlage, Shayla Murphy, and Kelsy Schoen for assistance with script writing and data collection. Thank you to the individuals who supported the Walk Across Kansas and raised funds to purchase equipment used in this project.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
REFERENCES
- 1.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainslie PN, Hoiland RL. Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol (1985) 117: 1081–1083, 2014. doi: 10.1152/japplphysiol.00854.2014. [DOI] [PubMed] [Google Scholar]
- 3.Alexandrov AV, Sloan MA, Wong LK, Douville C, Razumovsky AY, Koroshetz WJ, Kaps M, Tegeler CH; American Society of Neuroimaging Practice Guidelines Committee . Practice standards for transcranial Doppler ultrasound: part I–test performance. J Neuroimaging 17: 11–18, 2007. doi: 10.1111/j.1552-6569.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 4.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 5.Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol 133: 229–239, 2002. doi: 10.1016/S1569-9048(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 6.Behnke BJ, Delp MD. Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J Appl Physiol (1985) 108: 14–20, 2010. doi: 10.1152/japplphysiol.00970.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63, 2001. doi: 10.1016/S0034-5687(01)00195-5. [DOI] [PubMed] [Google Scholar]
- 8.Biasin L, Sage MD, Brunton K, Fraser J, Howe JA, Bayley M, Brooks D, McIlroy WE, Mansfield A, Inness EL. Integrating aerobic training within subacute stroke rehabilitation: a feasibility study. Phys Ther 94: 1796–1806, 2014. doi: 10.2522/ptj.20130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, Shaughnessy M, Tang A; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology . Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 2532–2553, 2014. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 10.Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G, Rippee MA. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther 36: 159–165, 2012. doi: 10.1097/NPT.0b013e318274d082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billinger SA, Sisante JV, Alqahtani AS, Pasnoor M, Kluding PM. Aerobic exercise improves measures of vascular health in diabetic peripheral neuropathy. Int J Neurosci 127: 80–85, 2017. doi: 10.3109/00207454.2016.1144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billinger SA, Sisante JV, Mattlage AE, Alqahtani AS, Abraham MG, Rymer MM, Camarata PJ. The relationship of pro-inflammatory markers to vascular endothelial function after acute stroke. Int J Neurosci 127: 486–492, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billinger SA, Tseng BY, Kluding PM. Modified total-body recumbent stepper exercise test for assessing peak oxygen consumption in people with chronic stroke. Phys Ther 88: 1188–1195, 2008. doi: 10.2522/ptj.20080072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 15.Briebach T, Laubenberger J, Fischer PA. Transcranial Doppler sonographic studies of cerebral autoregulation in Shy-Drager syndrome. J Neurol 236: 349–350, 1989. doi: 10.1007/BF00314378. [DOI] [PubMed] [Google Scholar]
- 16.Brothers RM, Zhang R. CrossTalk opposing view: The middle cerebral artery diameter does not change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4077–4079, 2016. doi: 10.1113/JP271884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, Pahigiannis K, Waddy SP, Koroshetz W. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol 36: 281–288, 2016. doi: 10.1007/s10571-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 117: 1090–1096, 2014. doi: 10.1152/japplphysiol.00285.2014. [DOI] [PubMed] [Google Scholar]
- 19.de la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3: 184–190, 2004. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- 20.Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol 533: 849–859, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32: 737–741, 1993. doi: 10.1227/00006123-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Giller CA, Giller AM, Cooper CR, Hatab MR. Evaluation of the cerebral hemodynamic response to rhythmic handgrip. J Appl Physiol (1985) 88: 2205–2213, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Giller CA, Hatab MR, Giller AM. Oscillations in cerebral blood flow detected with a transcranial Doppler index. J Cereb Blood Flow Metab 19: 452–459, 1999. doi: 10.1097/00004647-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 25.González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557: 331–342, 2004. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42: 2672–2713, 2011. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedlund S, Nylin G, Regnstrom O. The behaviour of the cerebral circulation during muscular exercise. Acta Physiol Scand 54: 316–324, 1962. doi: 10.1111/j.1748-1716.1962.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch S, Reichold J, Schneider M, Székely G, Weber B. Topology and hemodynamics of the cortical cerebrovascular system. J Cereb Blood Flow Metab 32: 952–967, 2012. doi: 10.1038/jcbfm.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoiland RL, Ainslie PN. CrossTalk proposal: the middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. J Physiol 594: 4073–4075, 2016. doi: 10.1113/JP271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber P. Angiographic evaluation of internal carotid blood flow in patients with cerebrovascular disease. Radiol Clin Biol 36: 82–90, 1967. [PubMed] [Google Scholar]
- 31.Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol (1985) 87: 1604–1608, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand 162: 13–20, 1998. doi: 10.1046/j.1365-201X.1998.0280f.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol Respir Environ Exerc Physiol 47: 954–960, 1979. [DOI] [PubMed] [Google Scholar]
- 34.Jørgensen LG, Perko G, Payne G, Secher NH. Effect of limb anesthesia on middle cerebral response to handgrip. Am J Physiol Heart Circ Physiol 264: H553–H559, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Jørgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol (1985) 73: 1825–1830, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Jørgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol (1985) 72: 1123–1132, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Jørgensen LG, Schroeder TV. Transcranial Doppler for detection of cerebral ischaemia during carotid endarterectomy. Eur J Vasc Surg 6: 142–147, 1992. doi: 10.1016/S0950-821X(05)80231-2. [DOI] [PubMed] [Google Scholar]
- 38.Jurca R, Jackson AS, LaMonte MJ, Morrow JR, Blair SN, Wareham NJ, Haskell WL, van Mechelen W, Church TS, Jakicic JM, Laukkanen R. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med 29: 185–193, 2005. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol (1985) 92: 2513–2520, 2002. doi: 10.1152/japplphysiol.01222.2001. [DOI] [PubMed] [Google Scholar]
- 40.Koga S, Rossiter HB, Heinonen I, Musch TI, Poole DC. Dynamic heterogeneity of exercising muscle blood flow and O2 utilization. Med Sci Sports Exerc 46: 860–876, 2014. doi: 10.1249/MSS.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 41.Lassen NA. Control of cerebral circulation in health and disease. Circ Res 34: 749–760, 1974. doi: 10.1161/01.RES.34.6.749. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Linkis P, Jørgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. J Appl Physiol (1985) 78: 12–16, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Looney MA, Rimmer JH. Aerobic exercise equipment preferences among older adults: a preliminary investigation. J Appl Meas 4: 43–58, 2003. [PubMed] [Google Scholar]
- 45.Lucas SJ, Cotter JD, Brassard P, Bailey DM. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cereb Blood Flow Metab 35: 902–911, 2015. doi: 10.1038/jcbfm.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mang CS, Brown KE, Neva JL, Snow NJ, Campbell KL, Boyd LA. Promoting motor cortical plasticity with acute aerobic exercise: a role for cerebellar circuits. Neural Plast 2016: 6797928, 2016. doi: 10.1155/2016/6797928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol (1985) 117: 1325–1336, 2014. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayhan WG, Arrick DM, Sun H, Patel KP. Exercise training restores impaired dilator responses of cerebral arterioles during chronic exposure to nicotine. J Appl Physiol (1985) 109: 1109–1114, 2010. doi: 10.1152/japplphysiol.00564.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moraine JJ, Lamotte M, Berré J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol 67: 35–38, 1993. doi: 10.1007/BF00377701. [DOI] [PubMed] [Google Scholar]
- 50.Pescatello LS; American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2014, p. xxiv. [Google Scholar]
- 51.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol 2: 933–996, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med 37: 765–782, 2007. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- 53.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol 23: 2055–2062, 2003. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 54.Roseguini BT, Davis MJ, Harold Laughlin M. Rapid vasodilation in isolated skeletal muscle arterioles: impact of branch order. Microcirculation 17: 83–93, 2010. doi: 10.1111/j.1549-8719.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589: 2847–2856, 2011. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. doi: 10.1161/01.STR.31.7.1672. [DOI] [PubMed] [Google Scholar]
- 57.Snow NJ, Mang CS, Roig M, McDonnell MN, Campbell KL, Boyd LA. The effect of an acute bout of moderate-intensity aerobic exercise on motor learning of a continuous tracking task. PLoS One 11: e0150039, 2016. doi: 10.1371/journal.pone.0150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res 24: 321–329, 1999. doi: 10.1023/A:1022534709672. [DOI] [PubMed] [Google Scholar]
- 59.Tarumi T, Zhang R. Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise. Front Physiol 5: 6, 2014. doi: 10.3389/fphys.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas SN, Schroeder T, Secher NH, Mitchell JH. Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol (1985) 67: 744–748, 1989. [DOI] [PubMed] [Google Scholar]
- 62.Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhäupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol 18: 1929–1934, 1997. [PMC free article] [PubMed] [Google Scholar]
- 63.Verbree J, Bronzwaer A, van Buchem MA, Daemen M, van Lieshout JJ, van Osch M. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab 2016 Jan 1 [Epub ahead of print]. doi: 10.1177/0271678X16679419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 117: 1084–1089, 2014. doi: 10.1152/japplphysiol.00651.2014. [DOI] [PubMed] [Google Scholar]
- 65.Vidoni ED, Yeh HW, Morris JK, Newell KL, Alqahtani A, Burns NC, Burns JM, Billinger SA. Cerebral β-amyloid angiopathy is associated with earlier dementia onset in Alzheimer’s disease. Neurodegener Dis 16: 218–224, 2016. doi: 10.1159/000441919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watts A, Honea RA, Billinger SA, Rhyner KT, Hutfles L, Vidoni ED, Burns JM. A combined measure of vascular risk for white matter lesions. J Alzheimers Dis 45: 187–193, 2015. doi: 10.3233/JAD-142085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whipp BJ. Peripheral chemoreceptor control of exercise hyperpnea in humans. Med Sci Sports Exerc 26: 337–347, 1994. doi: 10.1249/00005768-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol Respir Environ Exerc Physiol 52: 1506–1513, 1982. [DOI] [PubMed] [Google Scholar]
- 69.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–3275, 2012. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 592: 841–859, 2014. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]


