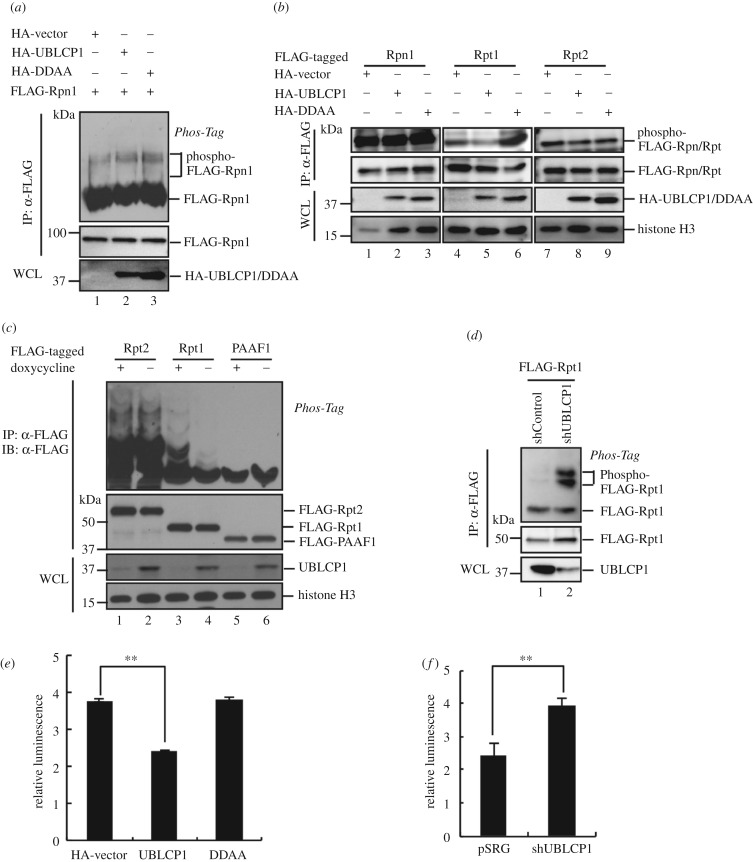
Figure 6.
UBLCP1 dephosphorylates Rpt1. (a) UBLCP1 does not alter Rpn1 phosphorylation. HEK293T cells were transfected with FLAG-Rpn1 and HA-UBCLP1 or DDAA mutant. After 48 h, cell lysates were subjected to immunoprecipitation using anti-FLAG antibody. IP products were probed with indicated antibodies. (b) UBLCP1, but not DDAA mutant, dephosphorylates Rpt1. HEK293T cells were transfected with FLAG-tagged proteasome subunits and HA-UBCLP1 or DDAA mutant. After 48 h, cell lysates were subjected to immunoprecipitation using anti-FLAG antibody. IP products were analysed with indicated antibodies. Phosphorylation levels of FLAG-tagged Rpn1, Rpt1 and Rpt2 were evaluated with an anti-phosphoserine antibody. (c) UBLCP1 removes up-shifted Rpt1 bands on Phos-tag gel. Samples were prepared as (a) from HeLa Tet-Off cell line with inducible UBLCP1 expression when doxycycline was removed. IP products were resolved on Phos-tag gel and SDS-PAGE, and then analysed by western blotting with indicated antibodies. (d) shUBLCP1 enhances Rpt1 phosphorylation on Phos-tag gel. Samples were prepared as (a). IP products were resolved on Phos-tag gel and SDS-PAGE, and then analysed by western blotting with indicated antibodies. (e) UBLCP1, but not DDAA, impairs Rpt1 ATPase activity in HEK293T cells. FLAG-Rpt1 was co-transfected with HA-UBLCP1 or DDAA mutant. After 48 h, cell lysates were subjected to immunoprecipitation using anti-FLAG antibody. IP products were incubated with ATP at 37°C for 30 min. ADP production was measured by ADP-Glo assay. Luminescence was read using a luminometer. All data are the means (±s.e.) of three independent experiments performed in triplicate; **p < 0.01, paired Student's t-test. (f) UBLCP1 knockdown enhances Rpt1 ATPase activity in HEK293T cells. FLAG-Rpt1 was transfected into shUBLCP1 and shControl cell lines. Samples were prepared and their ATPase activities were measured as in (e).