Fig. 5.
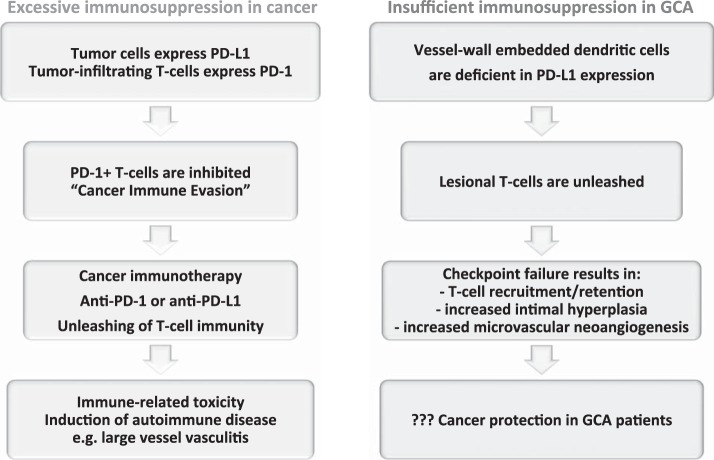
The PD-1 immune checkpoint in cancer and GCA interactions between PD-L1 and PD-1 provides negative signals to T cells, effectively suppressing T cell effector functions (see Fig. 2). Tumor cells escape from immune recognition by expressing the immunoinhibitory ligand PD-L1. T cells attempting to kill cancer cells are paralyzed by receiving negative signals through their PD-1 receptor. Recent breakthroughs in cancer immunotherapy capitalize on targeting PD-L1/PD-1 interactions. Checkpoint inhibitors prevent PD-L1/PD-1 interactions and have led to remarkable success in enhancing tumor immunity. The unleashing of T cell immunity in checkpoint-treated patients is associated with immune-related toxicity, e.g., the induction of autoimmune disease. Drug-induced vasculitis has been reported. In GCA, checkpoint signaling is impaired due to low PD-L1 expression on vessel wall DCs. The loss of this “immune break” enables PD-1+ T cells to infiltrate into the vessel wall and coordinate a wall-damaging inflammatory response. PD-1+ T cells have been implicated in regulating lesional cytokine production, driving microvascular neoangiogenesis, and enhancing lumen-occluding intimal hyperplasia. Given the deficiency of negative immune signaling implicated in cancer-immune evasion, GCA patients may have very effective antitumor immune responses.