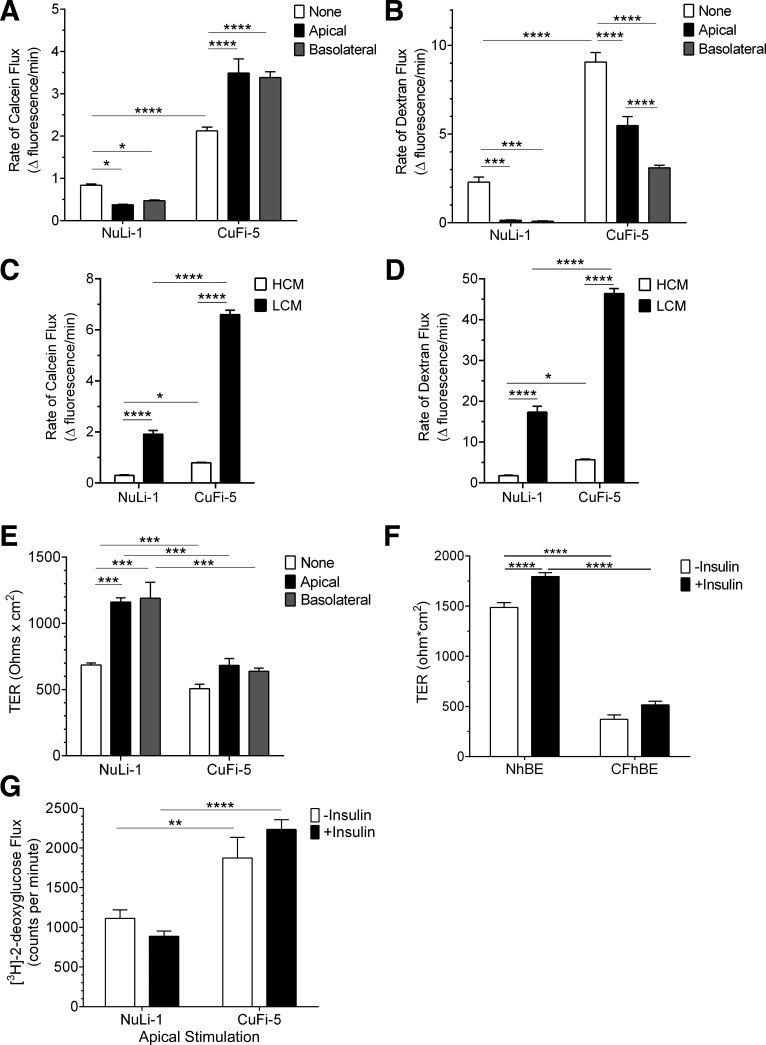
Fig. 7.
Insulin enhances normal airway epithelial barrier function but diminishes barrier function of F508del-CFTR airway cells. A: paracellular permeability flux measurements indicated that insulin (white bars, untreated; black bars, apical insulin; gray bars, basolateral insulin) significantly decreased flux of calcein in NuLi-1 cells while significantly increasing paracellular calcein flux in CuFi-5 cells. B: paracellular flux measurements of dextran indicated that both NuLi-1 and CuFi-5 decreased flux of dextran in response to insulin treatment. For both calcein and dextran paracellular flux rate datasets, n = 20 filters/condition. C and D: a calcium-switch assay was employed to assess the contribution of the paracellular barrier mediated by claudins to the flux of calcein (C) or dextran (D) through NuLi-1 and CuFi-5 monolayers. High-calcium media (HCM) demonstrate that CuFi-5 monolayers maintain a higher flux at baseline compared with NuLi-1 monolayers. E: in NuLi-1 cells, either apical or basolateral insulin treatment induced an increase in transepithelial resistance (TER) compared with untreated cells; n = 4 filters for each bar. F: in primary NhBE cells (n = 9), bilateral insulin treatment results in a significant increase in TER, whereas in primary CFhBE cells (n = 4) this increase does not occur. G: an apical solution containing insulin and 2-deoxy-d-[3H]glucose was applied for 60 min to measure glucose flux through both NuLi-1 and CuFi-5 monolayers on filters by scintillation counting; n = 12 filters for each bar. All data are shown as means ± SE and where *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 by unprotected two-way ANOVA Fisher’s LSD test.