Abstract
Key points
The integrity of the baroreflex control of sympathetic activity in heart failure (HF) remains under debate.
We proposed the use of the sequence method to assess the baroreflex control of renal sympathetic nerve activity (RSNA).
The sequence method assesses the spontaneous arterial pressure (AP) fluctuations and their related changes in heart rate (or other efferent responses), providing the sensitivity and the effectiveness of the baroreflex. Effectiveness refers to the fraction of spontaneous AP changes that elicits baroreflex‐mediated variations in the efferent response.
Using three different approaches, we showed that the baroreflex sensitivity between AP and RSNA is not altered in early HF rats. However, the sequence method provided evidence that the effectiveness of baroreflex in changing RSNA in response to AP changes is markedly decreased in HF.
The results help us better understand the baroreflex control of the sympathetic nerve activity.
Abstract
In heart failure (HF), the reflex control of the heart rate is known to be markedly impaired; however, the baroreceptor control of the sympathetic drive remains under debate. Applying the sequence method to a series of arterial pressure (AP) and renal sympathetic nerve activity (RSNA), we demonstrated a clear dysfunction in the baroreflex control of sympathetic activity in rats with early HF. We analysed the baroreflex control of the sympathetic drive using three different approaches: AP vs. RSNA curve, cross‐spectral analysis and sequence method between AP and RSNA. The sequence method also provides the baroreflex effectiveness index (BEI), which represents the percentage of AP ramps that actually produce a reflex response. The methods were applied to control rats and rats with HF induced by myocardial infarction. None of the methods employed to assess the sympathetic baroreflex gain were able to detect any differences between the control and the HF group. However, rats with HF exhibited a lower BEI compared to the controls. Moreover, an optimum delay of 1 beat was observed, i.e. 1 beat is required for the RSNA to respond after AP changing, which corroborates with the findings related to the timing between these two variables. For delay 1, the BEI of the controls was 0.45 ± 0.03, whereas the BEI of rats with HF was 0.29 ± 0.09 (P < 0.05). These data demonstrate that while the gain of the baroreflex is not affected in early HF, its effectiveness is markedly decreased. The analysis of the spontaneous changes in AP and RSNA using the sequence method provides novel insights into arterial baroreceptor reflex function.
Keywords: arterial baroreflex, heart failure, sequence method, sympathetic activity
Key points
The integrity of the baroreflex control of sympathetic activity in heart failure (HF) remains under debate.
We proposed the use of the sequence method to assess the baroreflex control of renal sympathetic nerve activity (RSNA).
The sequence method assesses the spontaneous arterial pressure (AP) fluctuations and their related changes in heart rate (or other efferent responses), providing the sensitivity and the effectiveness of the baroreflex. Effectiveness refers to the fraction of spontaneous AP changes that elicits baroreflex‐mediated variations in the efferent response.
Using three different approaches, we showed that the baroreflex sensitivity between AP and RSNA is not altered in early HF rats. However, the sequence method provided evidence that the effectiveness of baroreflex in changing RSNA in response to AP changes is markedly decreased in HF.
The results help us better understand the baroreflex control of the sympathetic nerve activity.
Abbreviations
- ANGII
angiotensin II
- AP
arterial pressure
- BEI
baroreflex effectiveness index
- +dP/dt
maximal slope of the systolic pressure increment
- −dP/dt
diastolic pressure decrement
- HF
heart failure
- HR
heart rate
- LVEDP
left ventricular end‐diastolic pressure
- MSNA
muscle sympathetic nerve activity
- NTS
nucleus tractus solitarii
- PI
pulse interval
- RSNA
renal sympathetic nerve activity
- SAP
systolic arterial pressure
- SAP50
systolic AP at 50% of RSNA range
Introduction
Heart failure (HF) is a global health problem with a high prevalence and is one of the leading worldwide causes of death (Roger, 2013). In HF, a complex interaction of several neurohumoral mechanisms become activated aiming to preserve cardiac function (Lymperopoulos et al. 2013). Among these mechanisms, sympathetic activation becomes a critical outcome in HF, markedly increasing its morbidity and mortality (Lymperopoulos et al. 2013). Sympathetic overactivity in HF has been evidenced by higher renal sympathetic nerve activity (RSNA) in rats and rabbits (Zucker & Wang, 1991; DiBona et al. 1995; Marcus et al. 2014; Ramchandra & Barrett, 2015) and an increase in muscle sympathetic nerve activity (MSNA) in patients (Leimbach et al. 1986; Grassi et al. 1995; Floras, 2001; Gronda et al. 2014). It is well established that the baroreflex control of the heart rate (HR) is markedly impaired in HF (Eckberg et al. 1971; Creager, 1992; Zucker et al. 2007). The loss of the inhibitory modulation by the arterial baroreflex has been proposed as a factor that is involved, at least in part, in the sustained sympathetic excitation in HF (Gronda et al. 2014). Although the baroreflex control of the HR is well known to be impaired in HF, the baroreceptor control of the sympathetic drive in this syndrome remains under debate. Dibner‐Dunlap et al. (1996) reported similar gains in the baroreflex control of MSNA in healthy and HF individuals. In experimental models of HF, preserved baroreflex control of RSNA was found in anaesthetized dogs with HF induced by pacing or aortovena‐caval fistula (Zucker et al. 1985; Dibner‐Dunlap & Thames, 1989). In contrast, reduced baroreflex control of sympathetic activity was observed in patients with HF (Ferguson et al. 1992; Grassi et al. 1995) and conscious rabbits with HF induced by pacing (Liu et al. 2000). Furthermore, using a cross‐spectral analysis, a similar transfer function (gain) between arterial pressure (AP) and MSNA was observed in healthy subjects and patients with HF, indicating that the baroreflex control of MSNA is preserved in this syndrome (Ando et al. 1997a,b).
The sequence method is an approach that assesses spontaneous AP fluctuations and their related changes in HR (Bertinieri et al. 1985). It is noteworthy that the sequence method provides information regarding the baroreflex function that is complementary to the baroreflex sensitivity (gain). The fraction of spontaneous AP ramps that effectively elicits baroreflex‐mediated changes in the HR or pulse interval (PI) provides an estimation of the baroreflex effectiveness index (BEI) (Di Rienzo et al. 2001). This index informs about the interaction between the arterial baroreflex and other non‐baroreflex mechanisms in controlling the heart. Most of the applications of the sequence method use pulse interval (or HR) signals as the efferent response to be analysed following AP changes (Laude et al. 2004; La Rovere et al. 2008). Although the sequence method has already been proven to be a useful tool for rats and mice (Stauss et al. 2006; Laude et al. 2008), it has never been applied when the RSNA was used as the efferent response signal in this models.
Therefore, considering the controversies regarding the baroreceptor control of the sympathetic drive in HF, we propose using the sequence method to assess the spontaneous baroreflex function, using AP and RSNA signals, as an alternative approach for analysing the baroreflex control of RSNA in rats with HF. In addition to the sequence method, two other methods were used to assess the baroreflex control: AP vs. RSNA curve and cross‐spectral analysis.
Methods
Ethical approval
All experimental procedures used in this study adhered to the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (Copyright © 1996 by the National Academy of Sciences), and were approved by the Committee of Ethics in Animal Research of the School of Medicine of Ribeirão Preto, University of São Paulo, SP, Brazil (Protocol no. 1477/2007). The authors understand the ethical principles under which The Journal of Physiology operates, and this work complies with the animal ethics checklist as described by Grundy (2015).
Animals
The experiments were carried out in adult male Wistar rats supplied by the Animal Facility of the University of São Paulo, Campus of Ribeirão Preto, SP, Brazil. The animals were housed individually with free access to food and water and were maintained on a 12 h light–dark cycle.
Experimental heart failure
Surgical ligation of the left coronary artery was performed to induce left ventricular dysfunction, which is the model of HF used in the present study (n = 6). Rats (250–300 g, 7–8 weeks old) were anaesthetized with ketamine (50 mg kg−1, i.p.) and xylazine (10 mg kg−1, i.p.). When the pedal withdrawal reflex was absent, the rats were endotracheally intubated and mechanically ventilated. A left thoracotomy was performed, and the left anterior descending coronary artery was ligated between the pulmonary artery outflow tract and the left atrium with a polyester suture (4–0; Ethicon, São José dos Campos, SP, Brazil). The control rats (n = 7) underwent a similar surgical procedure without the coronary ligation. All surgical procedures were carried out using aseptic techniques. After the surgery, analgesia was achieved (flunixin meglumine, 2.5 mg kg−1, i.m.), the temperature was controlled and the animals were maintained in a separate recovery area and monitored continuously until awake.
Recording of RSNA and AP
Four weeks after the coronary ligation, the animals were anaesthetized with ketamine (50 mg kg−1, i.p.) and xylazine (10 mg kg−1, i.p.). The surgical procedures were performed when the pedal withdrawal reflex was absent. The femoral artery and vein were catheterized with polyethylene tubing for the AP recording and intravenous drug administration. The right kidney was exposed via a retroperitoneal incision; the renal nerve was placed on a bipolar stainless steel pair of electrodes and secured with a polyvinyl siloxane impression material (Super‐Dent; Carlisle Laboratories, Rockville Centre, NY, USA). The electrode endings were exteriorized along with the vascular catheters in the animal's nape. All incisions were sutured, and the animal was allowed to recover in the recording room, with controlled temperature, where a quiet environment was maintained. Six to eight hours after the surgery, while the animals were awake and moved freely inside their cages, the arterial line was attached to a pressure transducer by (PE50) polyethylene tubing, and the renal nerve electrodes were connected to the recording system by flexible lightweight wires. No signals of distress were observed in either the control or HF rats during the experiments.
RSNA was properly amplified (×1000) and band‐pass filtered (100 Hz to 3 kHz) using a differential high‐impedance amplifier (CyberAmp; Axon Instruments, Union City, CA, USA) and digitally recorded (10 kHz) on an IBM/PC equipped with an analog‐to‐digital interface (DI220; ADInstruments, Akron, OH, USA). The AP was recorded simultaneously with RSNA using a pressure transducer (MLT844, ADInstruments, Bella Vista, Australia) attached to a blood pressure amplifier (Bridge Amp FE221, ADInstruments). After the basal recordings, the rats received a bolus injection of phenylephrine (4 μg kg−1, i.v., Sigma‐Aldrich, St Louis, MO, USA), which raised the AP, producing the complete disappearance of the RSNA. The minimal residual signal was considered the noise level. Then, sodium nitroprusside (32 μg kg−1, i.v., Sigma‐Aldrich) was administered to reduce AP, causing the renal sympathetic nerve to reach the maximal activity produced by baroreflex unloading.
The raw RSNA was rectified and integrated with a time constant of 10 ms, allowing the easy identification of the clusters of electrical activity that we call bursts. The number of bursts per minute during the basal period was used to measure the RSNA. After subtracting the background noise, RSNA was integrated every cardiac beat, normalized by the cardiac interval length, while its maximum and minimum activity were obtained using the peaks produced by sodium nitroprusside and phenylephrine, respectively. The beat‐by‐beat time series (5 min) of the systolic arterial pressure (SAP) and integrated RSNA were obtained for each animal. Hereafter, the integrated RSNA is simply referred to as RSNA. Those recordings were used to calculate the baroreflex function in HF and control rats.
At the end of the experimental protocol, the rats were anaesthetized with pentobarbital sodium (40 mg kg−1, i.p.) and a Mikro‐Tip catheter (Millar Instruments, Houston, TX, USA) was inserted into the left ventricle via the right carotid; the ventricular end‐diastolic pressure (LVEDP), maximal slope of the systolic pressure increment (+dP/dt) and diastolic pressure decrement (−dP/dt) were measured. At the completion of the experimental protocol, the rats were killed with an overdose of pentobarbital sodium (120 mg kg−1, i.p.). Then the heart was collected, weighed for calculation of the cardiac weight index (heart/body weight), and then submitted to histological processing. The infarct size (%) was determined by dividing the length of the infarcted area by the total size of the left ventricle in heart sections stained with picrosirius red (Lataro et al. 2013). HF was determined using both haemodynamic and morphological criteria. Only rats with an infarct size >40% of the cross‐sectional area of the left ventricle wall and an LVEDP ≥15 mmHg were enrolled in the study as previously described (Pfeffer et al. 1979; Xu et al. 2012).
Data analysis
Baroreflex function
The baroreflex creates a causal relationship between the AP and RSNA; thus, the baroreflex function can be assessed through the changes in RSNA elicited by the changes in the AP. Those changes in the AP can be spontaneous or induced by external stimuli. The baroreflex function curve (known as the barocurve) can be calculated when an external stimulus induces the AP to increase or decrease, covering a broad range of AP values. In contrast, the spontaneous fluctuations in AP have a much narrower range, and techniques for spontaneous variability might be employed, such as spectral and cross‐spectral analyses. In addition to the frequency methods, we propose using the sequence method for the AP and RSNA analysis.
Barocurve
The SAP vs. RSNA values were fitted by a four‐parameter non‐linear sigmoidal regression (Marquardt, 1963). The slope (gain) of the RSNA curve and the AP at the midpoint of the curve (SAP50) were calculated. To create a representative barocurve for each group, the fitted parameters were averaged for each group, and the mean parameters were used to create a representative sigmoidal curve.
Cross‐spectral analysis (transfer function)
The transfer function estimate is based on an input–output model of the physiological systems (Pinna & Maestri, 2001). In this case, SAP is considered the input and RSNA is considered the output of a linear time‐invariant system. In brief, the transfer function is calculated from the ratio of the RSNA‐SAP cross‐spectrum to the SAP spectrum (Porta et al. 2013), and its value represents the gain of the baroreflex. In addition to the transfer function, the (squared) coherence function between the input and output signals is frequently calculated; a 0.5 coherence threshold was chosen to discard the frequencies for which the transfer function estimate would not be reliable (Pinna & Maestri, 2001, 2002).
The original SAP and RSNA beat‐by‐beat series were converted to evenly spaced series using a cubic spline interpolation (10 Hz) and were divided into half‐overlapping segments of 512 data points (Welch, 1967). To attenuate the side effects, a Hanning window was used, and the spectrum of each segment was calculated using the fast Fourier transform (FFT). The transfer function (gain) and coherence between SAP and RSNA were integrated in low (LF: 0.2–0.8 Hz) and high frequency (HF: 0.8–3.0 Hz) bands, representing the Mayer's and respiratory oscillations of AP, respectively (Julien, 2008).
Sequence method
In contrast to the spectral approaches, the sequence method considers signals in the time domain. This method relies on the hypothesis that successive spontaneous increases or decreases (ramps) in the AP might elicit baroreflex‐mediated responses in either HR or sympathetic nerve activity. In most of the cases, the efferent signal used is the pulse interval (Parati et al. 2000; Laude et al. 2004). In the present study, the efferent signal considered is the RSNA.
The following five parameters are set in the sequence method: (1) the threshold for the AP values; (2) the threshold for the RSNA values (or other efferent variables, such as PI); (3) the threshold for the correlation coefficient of the sequence candidate (r); (4) the minimum sequence length (n); and (5) the delay between the AP and RSNA (or other efferent variables). Briefly, the sequence method searches for all ramps (up or down) of SAP that last at least n beats. The absolute differences between the successive values of SAP in all ramps must satisfy the threshold for the AP. Next, for each SAP ramp, the sequence method determines whether the values of RSNA, which are delayed by the number of points previously fixed, compose a ramp in the opposite direction to the SAP ramp. Thus, if SAP is increasing (decreasing), RSNA should be decreasing (increasing), considering the desired delay. The absolute differences between the successive values in the RSNA ramps must satisfy the threshold that was previously fixed. When the ramp of SAP matches the opposite RSNA ramp, a baroreflex sequence has been achieved. The gain of a particular sequence is calculated by the slope of the regression line of RSNA vs. SAP. If the correlation coefficient of the regression line is greater than the threshold r, the sequence is considered in the analysis. The final gain is the averaged slope, which was obtained from all the considered sequences.
In addition to the gain, the sequence method also provides an important index of baroreflex function, namely, BEI (Di Rienzo et al. 2001). BEI is simply the number of sequences divided by the number of AP ramps, interpreted as the percentage of AP ramps that are actually producing a reflex response by the baroreflex. BEI and gain are complementary indices of spontaneous baroreflex function, providing information on different aspects of baroreflex control (Di Rienzo et al. 2001).
According to previous studies in mice (Laude et al. 2008, 2009), the correlation threshold was set to r > 0.8, and the minimum sequence length was set to n = 3. Even though the use of thresholds for AP and PI are not encouraged by the referenced studies in mice, a pilot analysis conducted with the present dataset (obtained from rats) has demonstrated the need to use thresholds for SAP and RSNA, which were set to 0.5 mmHg and 0.5% RSNA, respectively. The delay between the systolic AP and RSNA, however, was not fixed. Instead, it was varied from 0 to 12 beats, and both the gain and BEI were calculated for each delay.
The same analyses were performed using surrogate data obtained from the original time series. The surrogate data were generated by shuffling both the SAP and RSNA time series, as proposed by Laude et al. (2008), which is a modification of the method proposed by Blaber et al. (1995).
Statistical analysis
The summary data are presented as the mean ± SEM. Two‐way ANOVA for repeated measurements, followed by the Holm–Sidak test, was performed to compare the gain and BEI for different delays between (1) the original and surrogate data, and (2) the control and HF rats. The Mann–Whitney rank sum test was employed to compare all the single variables. The level of significance was set at P < 0.05.
Results
Morphology and haemodynamics
The morphological and haemodynamic characteristics of the animals studied are summarized in Table 1. Control rats and rats with coronary ligation showed similar body weights, mean APs and HRs. However, the rats with coronary ligation exhibited a greater heart weight, cardiac weight index and LVEDP, and a lower +dP/dt.
Table 1.
Haemodynamic and morphological data from control and heart failure (HF) rats
| Control (n = 6) | HF (n = 6) | |||
|---|---|---|---|---|
| BW (g) | 494 | ±26 | 495 | ±15 |
| HW (g) | 1.30 | ±0.05 | 1.68 | ±0.12* |
| CWI (mg g−1) | 2.65 | ±0.1 | 3.23 | ±0.15* |
| Infarct size (%LV) | — | — | 46 | ±3 |
| LVEDP (mmHg) | 8 | ±1 | 17 | ±1* |
| +dP/dt (mmHg s−1) | 10,182 | ±284 | 6790 | ±338* |
| −dP/dt (mmHg s−1) | −10,363 | ±825 | −6470 | ±617 |
| MAP (mmHg) | 103 | ±2 | 107 | ±2 |
| HR (beats min−1) | 376 | ±15 | 395 | ±19 |
Data are means ± SEM. BW: body weight; HW: heart weight; CWI: cardiac weight index; LVEDP: left ventricle end‐diastolic pressure; +dP/dt and −dP/dt: maximal slope of the systolic increment and diastolic decrement of ventricular pressure, respectively; MAP: mean arterial pressure; HR: heart rate. * P < 0.05 compared to control group.
Renal sympathetic nerve activity
Figure 1 A, shows the original recordings of the AP, raw RSNA and integrated RSNA (every 10 ms) from control rats and rats with coronary ligation. The RSNA, calculated as bursts per minute (Fig. 1 B), was greater in the rats with coronary ligation compared with that in their control counterparts (434 ± 31 vs. 332 ± 31).
Figure 1. Renal sympathetic nerve activity (RSNA) in control rats (n = 7) and rats with heart failure (HF; n = 6).
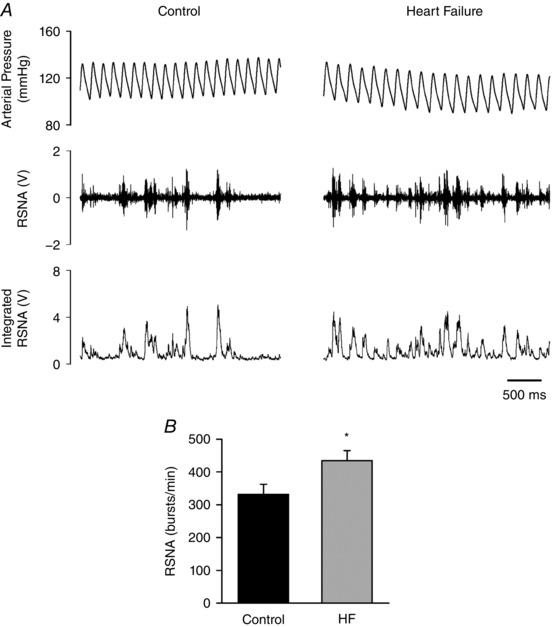
A, representative recordings of arterial pressure, raw RSNA and integrated RSNA. The integration was taken every 10 ms in this case. B, group data of bursts per minute. * P < 0.05 compared with control.
Barocurve
Figure 2 shows the average SAP vs. RSNA curves (barocurves) for the control rats and rats with coronary ligation. Note that both curves are virtually superimposed. The control rats and the rats with coronary ligation presented no differences in the baroreflex sensitivity (gain) and SAP50.
Figure 2. Mean baroreflex curves showing the relationship between SAP and RSNA fitted by a four‐parameters logistic sigmoidal regression.
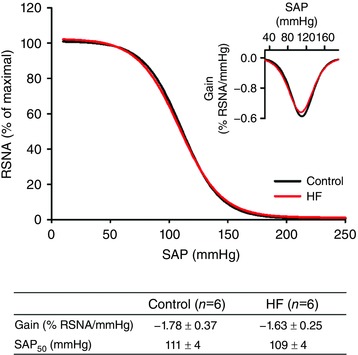
The inset shows the first derivative of the sigmoidal regression, and the table shows the group values of the parameters extracted from the barocurves. HF: heart failure; Gain: minimum value of the first derivate of the sigmoidal function; SAP50: SAP at the midpoint of the curve; no significant differences were found between the groups. [Color figure can be viewed at wileyonlinelibrary.com]
Cross‐spectral analysis
Figure 3 A shows an example of the coherence and transfer function spectra generated from a control animal recordin, while Fig. 3 B represents the group values of the baroreflex gain (transfer function). The gain was not different between the groups with respect to the low‐frequency (5.6 ± 1.6 vs. 11.2 ± 1.7% RSNA mmHg−1) or the high‐frequency (14.6 ± 4.7 vs. 15.2 ± 1.5% RSNA mmHg−1) band.
Figure 3. Representative examples of the coherence and transfer function (A) and the group values of baroreflex gain (B), calculated as the mean transfer function value, where coherence was >0.5.
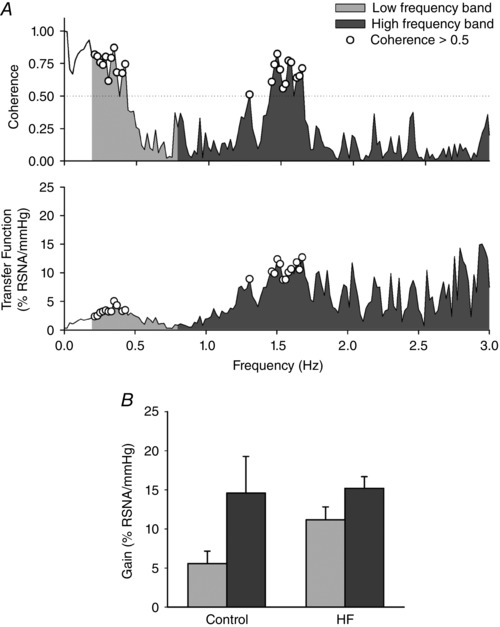
No significant differences were found between the control (n = 7) and HF (n = 6) rats. HF: heart failure.
Sequence method
Figure 4 shows the gain and BEI, calculated for delays ranging from 0 to 12 beats, for the control and HF groups. The results with the surrogate data are also presented. The baroreflex sensitivity, i.e. the gain, is not different between the control and HF groups for any delay. In contrast, the BEI is higher in the control group compared to that in the HF group for delays of 0, 1, 5, 6, 7, 9, 10 and 12 beats.
Figure 4. Gain (upper panels) and BEI (lower panels), calculated with the sequence method, for delays ranging from 0 to 12.
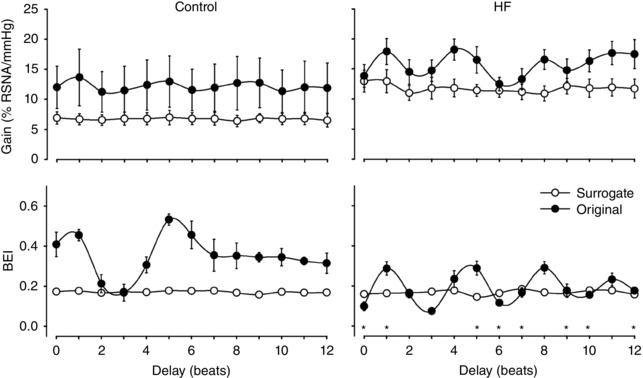
The results for the original (filled circles) and surrogate (open circles) series are shown in each plot. The left column shows the results for the control group (n = 7), and the right column shows the results for the HF group (n = 6). All results with the surrogate series (gain and BEI) showed no dependence on the delay. The BEI of both the control and HF groups (original data) significantly varied with the delay. HF: heart failure; * P < 0.05 compared with control group.
The results with the surrogate series (both gain and BEI) showed no dependence on delay (flat curves) for both the control and HF groups. In contrast, the BEI of the control and HF groups (original data) significantly changed with the delay. For delays of less than 5 beats, the BEI is maximized when a delay of 1 beat is assumed between the AP and RSNA (see Fig. 4). Considering the infeasibility of delays greater than 4 beats between the SAP and RSNA, a delay of 1 beat was chosen as the optimal delay.
Figure 5 shows the group values of the gain (Fig. 5 B) and BEI (Fig. 5 C) for delay 1 and a representative example of the calculation of the gain using the sequence method (Fig. 5 A). The gain is not different between the control and HF groups (13.6 ± 4.7 vs. 17.9 ± 2.1% RSNA mmHg−1, respectively). However, the BEI for the controls animals is significantly greater than that observed in rats with coronary ligation (0.45 ± 0.03 vs. 0.29 ± 0.03% RSNA mmHg−1, respectively).
Figure 5. Representative baroreflex sequences and group data of gain and BEI, calculated with the sequence method, for delay 1.
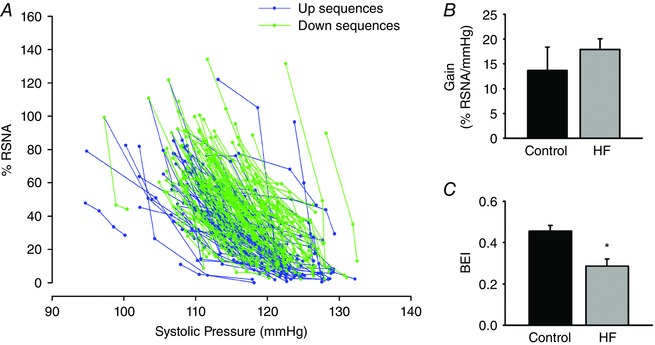
A, representative example of baroreflex sequences calculated with the sequence method using the parameters set in the Methods section and delay 1. B and C show the values of gain and BEI, respectively, for the control (n = 6) and HF (n = 7) groups. HF: heart failure; * P < 0.05 compared with control group. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
The analysis of the spontaneous baroreflex control has emerged as an important alternative to the classical pharmacological method of baroreflex assessment. While the former accounts for spontaneous AP fluctuations, the latter requires the injection of vasoactive drugs, which can produce undesirable effects, such as the stimulation of other reflexes or a direct stimulation on the sinus node (Parati et al. 2000). Another drawback of the pharmacological method is that higher and lower pressures, induced by the drugs, are not physiological situations and may not represent the system response under normal conditions.
Several methods have been proposed to assess the spontaneous baroreflex control (e.g. spectral analysis and the sequence method), and some studies have been conducted to compare their performances (Laude et al. 2004; Carrasco‐Sosa et al. 2005; Maestri et al. 2009). Spectral methods are based on the concept that spontaneous oscillations in AP elicit an oscillation at the same frequency in the efferent reflex response (cardiac interval, sympathetic nerve activity) (La Rovere et al. 2008). The advantage of the spectral analysis is the possibility to evaluate the baroreflex control of specific frequency bands of interest, such as low‐ (Mayer waves) or high‐frequency bands (respiration) (Parati et al. 2000; Pinna, 2007). The simplest baroreflex spectral analysis is the α‐index, which only considers the single power spectrum of AP and the reflex response (in this case, RSNA) and, therefore, assumes that the whole variability of the reflex response is driven by the AP (Faes et al. 2013).
In contrast, the transfer function method considers the power of the cross‐spectrum between the AP and RSNA. Thus, the baroreflex gain estimation is based only on the portion of the RSNA spectra shared between AP and RSNA, while disregarding the variability in RSNA due to sources other than the AP (Faes et al. 2013). However, some authors have drawn attention to the fact that both the α‐index and the transfer function approaches fail to fully represent the baroreflex closed loop because they represent only the feedback arm of the baroreflex and not the mechanical feed‐forward pathway. Thus, one drawback is that the estimated gain may be a mixture of both feedback and feed‐forward influences (Nollo et al. 2005; Faes et al. 2013; Porta et al. 2013). Another limitation of the spectral analysis is the assumption of a linear interaction between the AP and the efferent reflex variable (Nollo et al. 2005).
A coherence greater than 0.5 was adopted in this study as the criterion for gain estimation in the transfer function. Some authors have studied this issue (Pinna & Maestri, 2001) and proposed new criteria to reliably estimate gain (Pinna & Maestri, 2002). We also calculated the gain of transfer function without any coherence criteria, and the results were similar to those presented here.
Differing from spectral approaches, the sequence method searches for coherent changes in the AP and RSNA that are not limited to any definition of the frequency band (Parati et al. 2000). The correlation coefficient, calculated for each sequence candidate, plays a role similar to that of the coherence criteria in the transfer function. One advantage of the sequence method is the possibility to identify small segments (e.g. sequences of 3 or 4 beats), whereas spectral analysis decomposes the signals in long sinusoid components. A potential limitation of the sequence method occurs when high‐frequency oscillations are markedly present. As the values of pressure ramps must successively increase or decrease, the length of the sequences will be driven by any dominant frequency oscillation (e.g. respiration). Thus, although the sequence method is not restricted to any frequency band, the results may be limited to the oscillations produced in specific frequencies.
Baroreflex analysis with the sequence method is widely employed to investigate the cardiac baroreflex under clinical and experimental conditions. However, even under normal conditions, the rate of occurrence of baroreflex sequences (BEI) between AP and cardiac interval is relatively low (near 0.2) (Di Rienzo et al. 2001; Laude et al. 2008; Ariza et al. 2015; Rodrigues et al. 2015). Thus, the arterial baroreflex can respond to AP changes with concordant changes in cardiac interval in only a few (20%) cases. The presence of these uncoupled responses might indicate that, even in healthy subjects, the baroreflex, although it is continuously involved in cardiovascular homeostasis, may not be invariably effective in inducing reflex changes in response to AP transients.
To the best of our knowledge, this is the first study to apply the sequence methods to series of AP and RSNA in conscious rats. The first important finding of this study is that when RSNA is used as the reflex response variable, the BEI can reach much greater values compared to the values obtained using HR or cardiac interval, e.g. 0.45 vs. 0.2 (Di Rienzo et al. 2001). The observation of the limited influence of the baroreflex on the cardiac interval is an intriguing but well‐documented phenomenon (Wennergren et al. 1976; Di Rienzo et al. 2001; Laude et al. 2004; Abboud & Thames, 2011; Mancia & Mark, 2011). A possible explanation for the low BEI values may come from the observation that under physiological conditions, the cardiac rhythm and/or sympathetic drive are controlled not only by the baroreflex but also by other non‐baroreflex mechanisms (direct central neural influences, respiratory activity and humoral factors). It is plausible that the influences exerted by these non‐baroreflex mechanisms might be strong enough to overcome the baroreflex influence on the HR and/or sympathetic activity. Moreover, the baroreflex itself is known to be affected, to variable degrees, by central inhibitory influences (Wennergren et al. 1976), implying that the baroreflex function is a dynamic phenomenon. Therefore, the effectiveness of the baroreflex, quantified by the BEI, is likely to reflect the degree of the above interferences, i.e. those exerted by non‐baroreflex factors and those exerted by modulatory influences at a central level that control HR and/or sympathetic activity.
All approaches employed to assess baroreflex sensitivity/gain (barocurve, transfer function and the sequence method) failed to reveal any differences between the control rats and rats with HF. Interestingly, although the baroreflex gain is not affected in early HF, its effectiveness (BEI) is markedly reduced in rats with HF. Therefore, the impairment of the sympathetic baroreflex in HF seems to not be associated with the gain between AP and RSNA, but rather to be associated with the effectiveness of the baroreflex in changing the RSNA in response to the AP transients. The reduced BEI indicates a prevalence of non‐baroreflex mechanisms in animals with HF.
Several studies have noted that the cardiac BEI is decreased in pathological situations, such as diabetic neuropathy, arterial hypertension associated with chronic kidney disease and low level paraplegia (Johansson et al. 2005; Chan et al. 2008; Wang et al. 2012). Some diseases and the ageing process might induce changes at different levels of the nervous system, either inhibiting the baroreflex response or exacerbating non‐baroreflex mechanisms.
The activation of the renin–angiotensin–aldosterone system contributes to the pathogenesis of HF (Francis et al. 2001). Angiotensin II (ANGII) has been strongly implicated in the modulation of sympathetic outflow in the brain (Francis, 1989; Zucker et al. 2009). Clinical studies have also demonstrated that ANGII has an important sympathoexcitatory role in HF (Francis, 1989; Svanegaard et al. 1993). ANGII attenuates the baroreceptor reflex control of HR to increase, but not decrease, in AP (Campagnole‐Santos et al. 1988; Michelini & Bonagamba, 1990). This effect is mediated, largely, via the inhibition of vagal activation (Campagnole‐Santos et al. 1988). However, it has been demonstrated that baroreflex sympathoinhibition is also attenuated (Polson et al. 2007). In addition, it has been shown that the baroreflex inhibition by ANGII in the nucleus tractus solitarii (NTS) is dependent on GABAA receptors (Polson et al. 2007). Accordingly, inappropriately high levels of ANGII within the NTS may be a potential mechanism that affects the BEI after myocardial infarction. In addition, it is important to consider that the animals were studied 4 weeks after the myocardial infarction. The rats submitted to the left coronary artery ligation showed a larger infarct size, increased heart weight and LVEDP, and lower +dP/dt, which characterize the first stage of HF (Francis et al. 2001). Considering that HF exhibits a progressive decline in left ventricular function, we cannot exclude the possibility that the baroreflex gain in the control of RSNA could be reduced in the end stages of the disease. In addition, the changes in the autonomic cardiovascular control induced by the surgical stress should be considered since the experiment was performed 6–8 h after the catheter and electrode were implanted. However, the haemodynamic data obtained using this protocol do not show alterations reflecting a signal of distress.
An interesting feature of the BEI is its oscillatory behaviour as a function of the delay and the loss of these oscillations in the surrogate data (see Fig. 4). This oscillatory behaviour has already been reported with cardiac interval signals (Laude et al. 2008). Here, we showed that this behaviour is also present in the RSNA series, especially in the group of rats with early HF. At each 3 or 4 beats, the BEI reaches a maximum, creating an intriguing oscillatory pattern. We believe that this oscillation might reflect the periodic nature of the sequence occurrence.
Consider that the recordings (AP and RSNA), for some reason, tend to increase the percentage of the baroreflex sequences at each 3 or 4 beats. Respiration, for example, can create this type of oscillation because the respiratory influence on SAP and PI will limit the occurrence of ramps, depending on the respiratory frequency. Thus, for a given SAP ramp, one can associate several PI ramps, varying the delay, and the association can be greater or lower depending on the position of the signal where more PI ramps were found. However, for higher delays, the association between SAP and PI is spurious because the PI ramp is actually elicited by an SAP ramp prior to the given SAP ramp. Therefore, using a fixed SAP ramp, one can find many PI ramps associated with it, which can be several delays far from the SAP ramp, but these PI ramps may not be associated with a physiological condition. The same logic is applicable to SAP and RSNA.
We defined the best delay between SAP and RSNA as 1 beat. Although the highest BEI in the control group is observed at delay 5, we believe that five heart beats (approximately 800 ms) is too much time for the interaction between SAP and RSNA. Moreover, other authors reported that the timing between the stimulation of the aortic depressor nerve and the response in RSNA is 100 ms, which approximates a delay of 1 beat (Petiot et al. 2001). Therefore, the higher BEI at delay 5 might be related to the effect of the oscillatory behaviour previously discussed. In the HF group, delays 1 and 5 also present the highest values, although the oscillatory pattern is much clearer, and the values are lower, compared to those in the control group.
Conclusions
We propose using the sequence method as an additional approach to analysing the baroreflex function with a beat‐by‐beat series of AP and RSNA. While the gain of the baroreflex, calculated using three different approaches, did not reveal any differences between the control rats and rats with HF, the BEI is markedly decreased in early HF. Hence, regarding the examination of the baroreflex control of the sympathetic drive, it is also important to consider the baroreflex effectiveness (BEI) in addition to the assessment of the baroreflex sensitivity/gain. A delay of 1 beat between AP and RSNA seems to be the best timing between these two variables. Moreover, the applicability of this approach in humans is straightforward, and the simultaneous recording of SNA and AP can be performed. The application of the sequence method in these signals provided novel insights into the arterial baroreceptor reflex function in HF and may contribute to the understanding of the baroreflex control of sympathetic nerve activity in health and other diseases.
Additional information
Competing interests
None declared.
Author contributions
R.M.L., L.E.V.S., C.A.A.S., H.C.S. and R.F. were responsible for study conception and experimental design; R.M.L. and C.A.A.S. performed the experiments; R.M.L., L.E.V.S. and R.F. analysed and interpreted the data; R.M.L., L.E.V.S., H.C.S. and R.F. drafted the article; and R.M.L., L.E.V.S., C.A.A.S., H.C.S. and R.F. revised the article critically for important intellectual content. All authors have approved the final version of the manuscript and agreed to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Acknowledgements
The authors thank Rubens F. de Melo for the excellent histological support.
References
- Abboud FM & Thames MD (2011). Interaction of cardiovascular reflexes in circulatory control In Comprehensive Physiology. John Wiley & Sons, Inc; Available at: https://doi.org/10.1002/cphy.cp020319. [Google Scholar]
- Ando S, Dajani HR & Floras JS (1997a). Frequency domain characteristics of muscle sympathetic nerve activity in heart failure and healthy humans. Am J Physiol 273, R205–R212. [DOI] [PubMed] [Google Scholar]
- Ando S, Dajani HR, Senn BL, Newton GE & Floras JS (1997b). Sympathetic alternans. Evidence for arterial baroreflex control of muscle sympathetic nerve activity in congestive heart failure. Circulation 95, 316–319. [DOI] [PubMed] [Google Scholar]
- Ariza D, Sisdeli L, Crestani CC, Fazan R & Martins‐Pinge MC (2015). Dysautonomias in Parkinson's disease: cardiovascular changes and autonomic modulation in conscious rats after infusion of bilateral 6‐OHDA in substantia nigra. Am J Physiol Heart Circ Physiol 308, H250–H257. [DOI] [PubMed] [Google Scholar]
- Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A & Mancia G (1985). A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3, S79–S81. [PubMed] [Google Scholar]
- Blaber AP, Yamamoto Y & Hughson RL (1995). Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol 268, H1682–H1687. [DOI] [PubMed] [Google Scholar]
- Campagnole‐Santos MJ, Diz DI & Ferrario CM (1988). Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension 11, I167–I171. [DOI] [PubMed] [Google Scholar]
- Carrasco‐Sosa S, Gaitán‐González MJ, González‐Camarena R & Yáñez‐Suárez O (2005). Baroreflex sensitivity assessment and heart rate variability: relation to maneuver and technique. Eur J Appl Physiol 95, 265–275. [DOI] [PubMed] [Google Scholar]
- Chan CT, Shen XS, Picton P & Floras J (2008). Nocturnal home hemodialysis improves baroreflex effectiveness index of end‐stage renal disease patients. J Hypertens 26, 1795–1800. [DOI] [PubMed] [Google Scholar]
- Creager MA (1992). Baroreceptor reflex function in congestive heart failure. Am J Cardiol 69, 10G–15G; discussion 15G–16G. [DOI] [PubMed] [Google Scholar]
- Dibner‐Dunlap ME, Smith ML, Kinugawa T & Thames MD (1996). Enalaprilat augments arterial and cardiopulmonary baroreflex control of sympathetic nerve activity in patients with heart failure. J Am Coll Cardiol 27, 358–364. [DOI] [PubMed] [Google Scholar]
- Dibner‐Dunlap ME & Thames MD (1989). Baroreflex control of renal sympathetic nerve activity is preserved in heart failure despite reduced arterial baroreceptor sensitivity. Circ Res 65, 1526–1535. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Jones SY & Brooks VL (1995). ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol 269, R1189–R1196. [DOI] [PubMed] [Google Scholar]
- Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G & Pedotti A (2001). Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol 280, R744–R751. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Drabinsky M & Braunwald E (1971). Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 285, 877–883. [DOI] [PubMed] [Google Scholar]
- Faes L, Masè M, Nollo G, Chon KH & Florian JP (2013). Measuring postural‐related changes of spontaneous baroreflex sensitivity after repeated long‐duration diving: frequency domain approaches. Auton Neurosci 178, 96–102. [DOI] [PubMed] [Google Scholar]
- Ferguson DW, Berg WJ, Roach PJ, Oren RM & Mark AL (1992). Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 69, 523–531. [DOI] [PubMed] [Google Scholar]
- Floras JS (2001). Arterial baroreceptor and cardiopulmonary reflex control of sympathetic outflow in human heart failure. Ann NY Acad Sci 940, 500–513. [DOI] [PubMed] [Google Scholar]
- Francis GS (1989). The relationship of the sympathetic nervous system and the renin‐angiotensin system in congestive heart failure. Am Heart J 118, 642–648. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei SG, Johnson AK & Felder RB (2001). Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281, R1734–R1745. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB & Pozzi M (1995). Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92, 3206–3211. [DOI] [PubMed] [Google Scholar]
- Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, Lovett EG, Mancia G & Grassi G (2014). Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof‐of‐concept study. Eur J Heart Fail 16, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Gao SA, Friberg P, Annerstedt M, Bergström G, Carlström J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen F‐D & Strömbom U (2005). Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens 18, 995–1000; discussion 1016. [DOI] [PubMed] [Google Scholar]
- Julien C (2008). Baroreflex control of sympathetic nerve activity and blood pressure variability. Clin Exp Pharmacol Physiol 35, 512–515. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD & Raczak G (2008). Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13, 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataro RM, Silva CAA, Fazan R, Rossi MA, Prado CM, Godinho RO & Salgado HC (2013). Increase in parasympathetic tone by pyridostigmine prevents ventricular dysfunction during the onset of heart failure. Am J Physiol Regul Integr Comp Physiol 305, R908–R916. [DOI] [PubMed] [Google Scholar]
- Laude D, Elghozi JL, Girard A, Bellard E, Bouhaddi M, Castiglioni P, Cerutti C, Cividjian A, Di Rienzo M, Fortrat JO, Janssen B, Karemaker JM, Lefthériotis G, Parati G, Persson PB, Porta A, Quintin L, Regnard J, Rüdiger H & Stauss HM (2004). Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study). Am J Physiol Regul Integr Comp Physiol 286, R226–R231. [DOI] [PubMed] [Google Scholar]
- Laude D, Baudrie V & Elghozi J‐L (2008). Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am J Physiol Regul Integr Comp Physiol 294, R142–R150. [DOI] [PubMed] [Google Scholar]
- Laude D, Baudrie V & Elghozi J‐L (2009). Tuning of the sequence technique. IEEE Eng Med Biol Mag 28, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimbach WN, Wallin BG, Victor RG, Aylward PE, Sundlöf G & Mark AL (1986). Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73, 913–919. [DOI] [PubMed] [Google Scholar]
- Liu JL, Irvine S, Reid IA, Patel KP & Zucker IH (2000). Chronic exercise reduces sympathetic nerve activity in rabbits with pacing‐induced heart failure: A role for angiotensin II. Circulation 102, 1854–1862. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G & Koch WJ (2013). Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 113, 739–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri R, Raczak G, Torunski A, Sukiennik A, Kozłowski D, La Rovere MT & Pinna GD (2009). Day‐by‐day variability of spontaneous baroreflex sensitivity measurements: implications for their reliability in clinical and research applications. J Hypertens 27, 806–812. [DOI] [PubMed] [Google Scholar]
- Mancia G & Mark AL (2011). Arterial baroreflexes in humans In Comprehensive Physiology. John Wiley & Sons, Inc; Available at: https://doi.org/10.1002/cphy.cp020320. [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia X‐H & Schultz HD (2014). Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt D (1963). An algorithm for least‐squares estimation of nonlinear parameters. J Soc Indust Appl Math 11, 431–441. [Google Scholar]
- Michelini LC & Bonagamba LG (1990). Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension 15, I45–I50. [DOI] [PubMed] [Google Scholar]
- Nollo G, Faes L, Porta A, Antolini R & Ravelli F (2005). Exploring directionality in spontaneous heart period and systolic pressure variability interactions in humans: implications in the evaluation of baroreflex gain. Am J Physiol Heart Circ Physiol 288, H1777–H1785. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M & Mancia G (2000). How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens 18, 7–19. [PubMed] [Google Scholar]
- Petiot E, Barrès C, Chapuis B & Julien C (2001). Frequency response of renal sympathetic nervous activity to aortic depressor nerve stimulation in the anaesthetized rat. J Physiol 537, 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA & Braunwald E (1979). Myocardial infarct size and ventricular function in rats. Circ Res 44, 503–512. [DOI] [PubMed] [Google Scholar]
- Pinna GD (2007). Assessing baroreflex sensitivity by the transfer function method: what are we really measuring? J Appl Physiol 1985 102, 1310–1311. [DOI] [PubMed] [Google Scholar]
- Pinna GD & Maestri R (2001). Reliability of transfer function estimates in cardiovascular variability analysis. Med Biol Eng Comput 39, 338–347. [DOI] [PubMed] [Google Scholar]
- Pinna GD & Maestri R (2002). New criteria for estimating baroreflex sensitivity using the transfer function method. Med Biol Eng Comput 40, 79–84. [DOI] [PubMed] [Google Scholar]
- Polson JW, Dampney RAL, Boscan P, Pickering AE & Paton JFR (2007). Differential baroreflex control of sympathetic drive by angiotensin II in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol 293, R1954–R1960. [DOI] [PubMed] [Google Scholar]
- Porta A, Bari V, Bassani T, Marchi A, Pistuddi V & Ranucci M (2013). Model‐based causal closed‐loop approach to the estimate of baroreflex sensitivity during propofol anesthesia in patients undergoing coronary artery bypass graft. J Appl Physiol 1985 115, 1032–1042. [DOI] [PubMed] [Google Scholar]
- Ramchandra R & Barrett CJ (2015). Regulation of the renal sympathetic nerves in heart failure. Front Physiol 6, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues FL, Silva LEV, Hott SC, Bomfim GF, da Silva CAA, Fazan R, Resstel LBM, Tostes RC & Carneiro FS (2015). Toll‐like receptor 9 plays a key role in the autonomic cardiac and baroreflex control of arterial pressure. Am J Physiol Regul Integr Comp Physiol 308, R714–R723. [DOI] [PubMed] [Google Scholar]
- Roger VL (2013). Epidemiology of heart failure. Circ Res 113, 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss HM, Moffitt JA, Chapleau MW, Abboud FM & Johnson AK (2006). Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291, H482–H483. [DOI] [PubMed] [Google Scholar]
- Svanegaard J, Johansen JB, Thayssen P & Haghfelt T (1993). Neurohormonal systems during progression of heart failure: a review. Cardiology 83, 21–29. [DOI] [PubMed] [Google Scholar]
- Wang S, Randall DC, Knapp CF, Patwardhan AR, Nelson KR, Karounos DG & Evans JM (2012). Blood pressure regulation in diabetic patients with and without peripheral neuropathy. Am J Physiol Regul Integr Comp Physiol 302, R541–R550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P (1967). The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 15, 70–73. [Google Scholar]
- Wennergren G, Little R & Oberg B (1976). Studies on the central integration of excitatory chemoreceptor influences and inhibitory baroreceptor and cardiac receptor influences. Acta Physiol Scand 96, 1–18. [DOI] [PubMed] [Google Scholar]
- Xu B, Zheng H & Patel KP (2012). Enhanced activation of RVLM‐projecting PVN neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 302, H1700–H1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Gorman AJ, Cornish KG & Lang M (1985). Impaired atrial receptor modulation or renal nerve activity in dogs with chronic volume overload. Cardiovasc Res 19, 411–418. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD & Rossing MA (2007). Chronic baroreceptor activation enhances survival in dogs with pacing‐induced heart failure. Hypertension 50, 904–910. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Schultz HD, Patel KP, Wang W & Gao L (2009). Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol 297, H1557–H1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH & Wang W (1991). Reflex control of renal sympathetic nervous activity in heart failure. Herz 16, 82–91. [PubMed] [Google Scholar]


