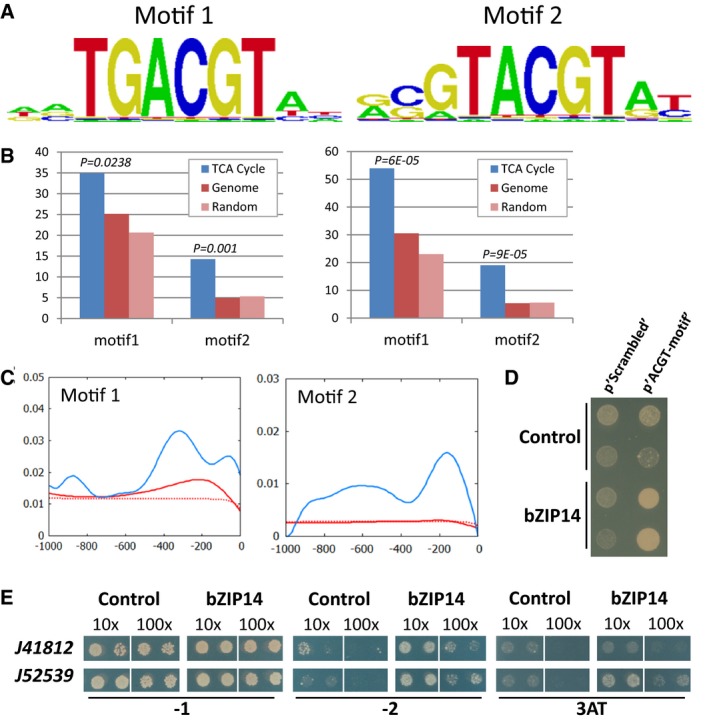
TF binding sites as predicted by the protein binding array represented as positional weight matrixes. Shown are the two binding motifs obtained by generating the consensus motif from all oligonucleotides bound by the recombinant protein with an E‐score > 0.45.
Scan for the bZIP14 motifs in all P. tricornutum gene promoters (genome, in red; random promoters, in light red) or only those linked to the TCA cycle (in blue). Left and right panels show the % promoters with motif 1 or 2 and the number of motifs per promoter, respectively, within 0.5 kb upstream of the ORF. P‐values correspond to the comparison between the promoters of the TCA cycle containing the motif and all the promoters of the genome containing the same motif (hypergeometric distribution).
Histogram showing an increased density of the bZIP14 motifs in the proximal promoter regions of the “TCA cycle genes” (in blue), compared with those in the whole genome (in red) or in random promoters (dashed red). The complete scan of 1.0 kb upstream of the ORF is shown.
Y1H analysis with a synthetic promoter. The full‐length bZIP14 ORF fused to GAL4AD (bZIP14) or the empty vector control (control) was expressed in reporter strains harbouring the HIS3 gene under control of a synthetic promoter element consisting of both bZIP14 motif1 and motif2 with random nucleotide spacers (p'ACGT‐motif') or a scrambled version (p'Scrambled') thereof. Transformed yeast cultures dropped in serial dilutions (10‐ and 100‐fold) were grown for 6 days on selective medium (minus histidine and plus 3‐AT).
Y1H analysis with diatom gene promoters. The full‐length bZIP14 ORF fused to GAL4AD (bZIP14) or the empty vector control (control) was expressed in reporter strains harbouring the HIS3 gene under control of a 150‐bp and 219‐bp promoter sequence of the P. tricornutum genes Phatr3_J41812 and (mitochondrial succinate dehydrogenase iron‐sulphur subunit) Phatr3_J52539, respectively. Transformed yeast cultures (two independent transformants) dropped in serial dilutions (10‐ and 100‐fold) were grown for 6 days on control medium (−1) and selective medium (minus histidine (−2) or minus histidine plus 3‐AT (3AT)).