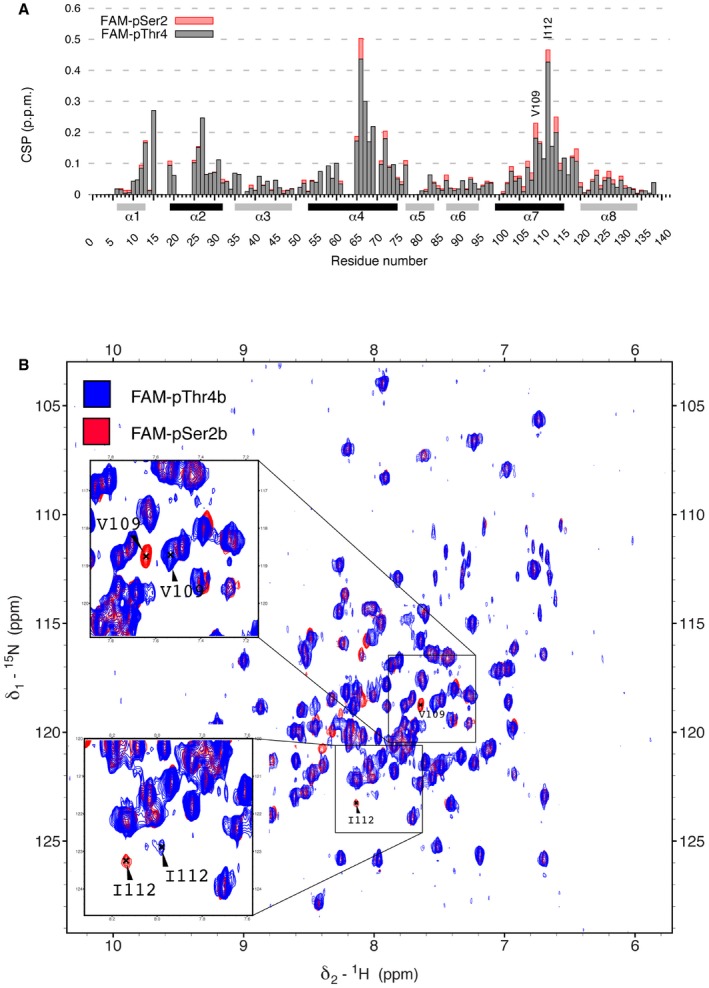
In order to identify the surface of the CID involved in interaction with pThr4‐CTD, we performed a
1H
15N‐TROSY NMR titration experiment, where
15N‐labelled Rtt103 CID was titrated with pThr4 peptide. Titration confirmed that Rtt103p CID is interacting with pThr4‐CTD peptide in an almost identical fashion as with pSer2‐CTD, using the canonical surface of helices α2, α4 and α7 (Fig
1B and C). However, important rearrangements were observed for Val109 and Ile112, residues that lay in close proximity of P
3b and T
4b.
Chemical shift perturbations (CSP) of the Rtt103p CID upon interaction with FAM‐pSer2 CTD (red) or FAM‐pThr4 CTD (grey) peptides plotted against residue number of Rtt103p CID. Secondary structure elements are shown below the x‐axis. Helices involved in the interaction with phospho‐peptides are coloured in black. FAM, 5,6‐carboxyfluorescein.
Overlay of 1H‐15N TROSY spectra of complex of Rtt103p CID with FAM‐pSer2 (red) and FAM‐pThr4 (blue).