Figure 1. Mis18BP120–130 is sufficient to interact with Mis18α/β.
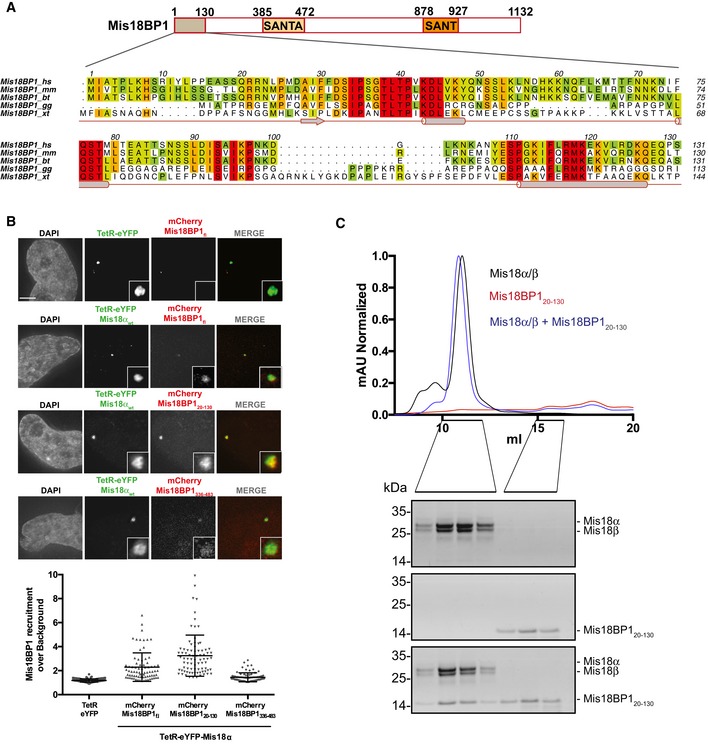
- Schematic representation of Mis18BP1 domain architecture, amino acid conservation (conservation score is mapped from red to cyan, where red corresponds to highly conserved and cyan to poorly conserved), and secondary structure (Conserved Domain Database [CDD] and PsiPred, http://bioinf.cs.ucl.ac.uk/psipred). Alignments include Homo sapiens (hs), Mus musculus (mm), Bos taurus (bt), Gallus gallus (gg), and Xenopus tropicalis (xt). Multiple sequence alignments were performed with MUSCLE 31 and edited with Aline 32.
- Representative fluorescence images (top) and quantification (bottom) for the analysis of mCherry‐Mis18BP1 recruitment to the alphoidtetO array by TetR‐eYFP‐Mis18α. HeLa 3‐8 cells co‐transfected with either TetR‐eYFP or TetR‐eYFP‐Mis18αwt and mCherry vectors containing the indicated versions of Mis18BP1. Middle bars show median whilst error bars show SEM. Mann–Whitney test vs. TetR‐eYFP; P ≤ 0.0001, n ≥ 77. Scale bar, 5 μm.
- SEC profiles and respective SDS–PAGE analysis of Mis18α/β, Mis18BP120–130, and Mis18α/β mixed with molar excess of Mis18BP120–130.
