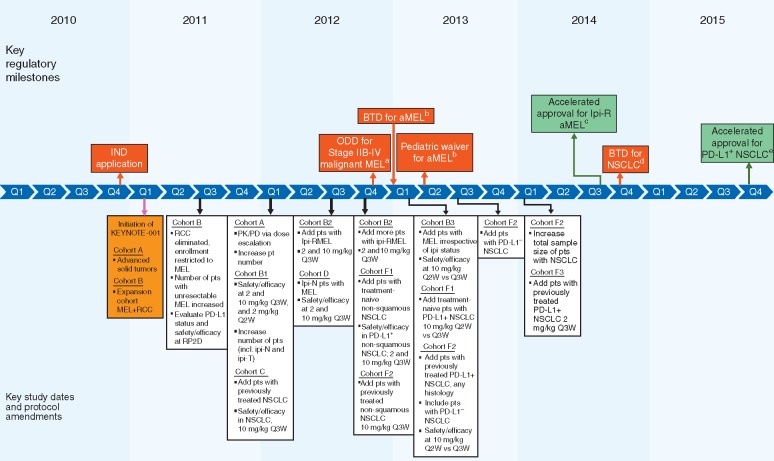
Figure 2.
KEYNOTE-001: timeline of key study design elements and US FDA regulatory milestones. ALK, anaplastic lymphoma kinase; aMEL, metastatic melanoma; BTD, breakthrough therapy designation; chemo, chemotherapy; EGFR, epidermal growth factor receptor; incl., including; IND, investigational new drug; Ipi(-R, -T, -N), ipilimumab (-refractory, -treated, -naive); MEL, melanoma; NSCLC, non-small cell lung cancer; ODD, orphan drug designation; OL, open label; PD, pharmacodynamics; PD-L1+, positive for expression of programmed death ligand 1; PD-L1–, negative for expression of programmed death ligand 1; PK, pharmacokinetic; pts, patients; Q2W, every 2 weeks; Q3W, every 3 weeks; RCC, renal cell carcinoma; RP2D, recommended phase 2 dose; US FDA, United States Food and Drug Administration. aFDA granted ODD for stage IIB-IV malignant melanoma [24]. bFDA granted BTD for advanced melanoma and a pediatric waiver based on ODD status [25]. cFDA granted accelerated approval for unresectable or metastatic melanoma and disease progression after ipilimumab and, if BRAFV600 mutation positive, a BRAF inhibitor; approved dose 2 mg/kg Q3W [23]. dFDA granted BTD for treatment of EGFR mutation negative and ALK rearrangement-negative NSCLC with disease progression on or after platinum-based chemotherapy [28]. eFDA granted accelerated approval for metastatic NSCLC with tumors expressing PD-L1 (as determined by an FDA-approved test) and with disease progression on or after platinum-containing chemotherapy (EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations before receiving pembrolizumab); approved dose 2 mg/kg Q3W [29].