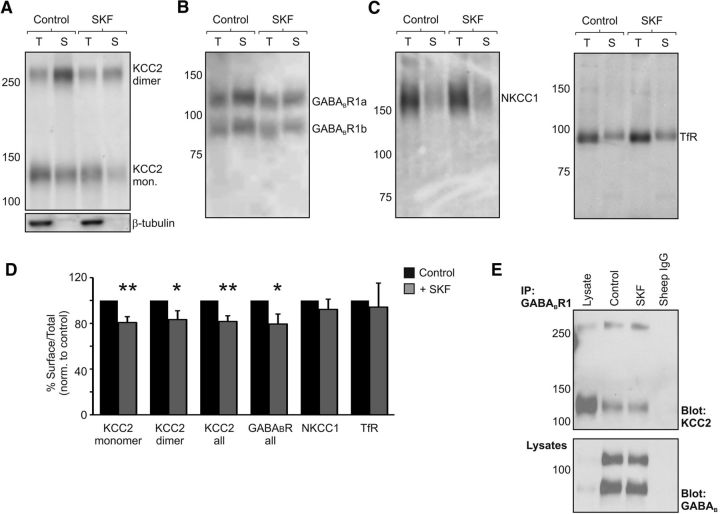
Figure 5.
GABABR activation leads to a reduction in KCC2 at the membrane surface. A, GABABR activation leads to a reduction in the fraction of both monomeric and dimeric forms of KCC2 at the membrane surface. Rat organotypic hippocampal slices (P7 + 7–14 DIV) were exposed to normal ACSF (Control) or ACSF containing SKF97541 for 20 min (SKF, 5 μm), and then biotinylated to label proteins at the cell surface. Cell homogenates of total protein (T) and streptavidin-purified cell surface proteins (S) were probed on Western blots with anti-KCC2 antibodies (top). Lack of β-tubulin staining in surface samples confirmed that the cell membranes remained intact (bottom). B, SKF97541 treatment also led to a concomitant reduction in surface levels of GABABR1 relative to controls. C, Neither surface-bound NKCC1 nor the surface levels of the transferrin receptor changed following GABABR activation. D, Summary plot of the effect of GABABR activation upon proteins at the membrane surface after 20 min 5 μm SKF97541 treatment. Surface expression was quantified as the ratio of surface to total protein, normalized to control slices that were examined in parallel. SKF97541 treatment resulted in a significant reduction in the surface ratio for KCC2 (monomer, **p = 0.002; dimer, *p = 0.048; all, **p = 0.003; n = 14, t test) and GABABR1 (all, *p = 0.046, n = 9, t test). No effect was observed in the surface/total ratio for NKCC1 (p = 0.4, n = 13, t test) or the transferrin receptor (TfR; p = 0.79, n = 8, t test). E, The association between KCC2 and GABABR remains following SKF97541 treatment. Rat organotypic hippocampal slices were solubilized and subjected to coimmunoprecipitation analysis using GABABR1 as the precipitating antibody. The amount of KCC2 in GABABR1 complexes (top), normalized by the amount of GABABR1 protein precipitated (bottom), did not change following SKF97541 treatment (p = 0.44, n = 3, t test).