Figure 8.
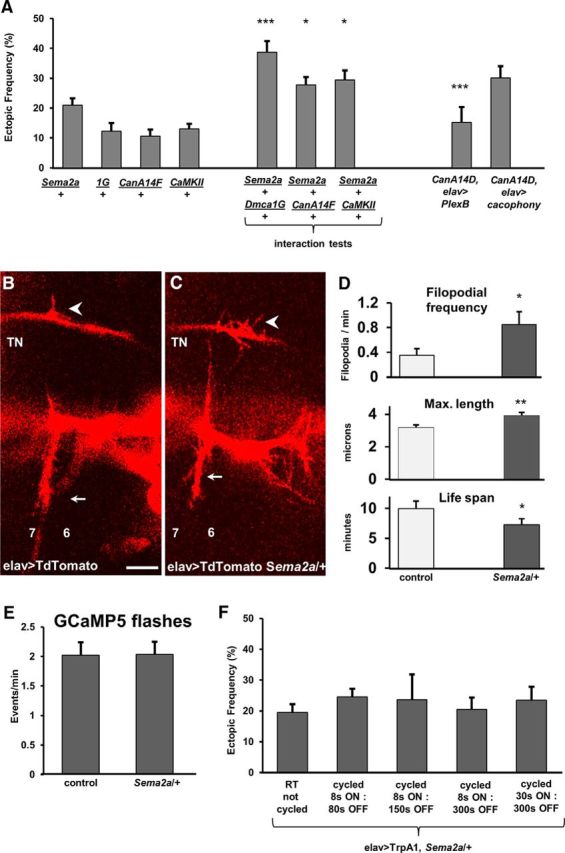
CanA and CaMKII may function in a pathway that involves Ca2+ signaling and Sema2a-dependent chemorepulsion for refinement. A, Genetic interactions were observed between heterozygous mutants for the trans-synaptic chemorepellant sema2a and heterozygotes for Dmca1G, CanA14F, and CaMKII. Rescue experiments by the pan-neural expression of PlexB (CanA14D/>;;UAS-PlexinB/elavGS-GAL4,UAS-CD8-GFP) or Cacophony (CanA14D/+;;UAS-cacophony/elavGAL4,UAS-TdTomato) in CanA14D mutants (n = 34, 18, 25, 18, 17, 13, 27, 11, and 11, respectively; p = 0.0002, 0.03, 0.02, and 0.0002, respectively). B, C, Motoneuron filopodia from growth cones on the TN and SNb nerve in a Stage 17a Mhc1 embryo (16.5 h AEL) are shown as revealed by pan-neural TdTomato expression in a control animal (B) and a heterozygous Sema2a mutant (C). Arrow indicates ectopic filopodial contact. Arrowhead indicates native innervation. Muscles 7 and 6 are labeled for orientation. D, In vivo imaging of fluorescently tagged filopodia in intact embryos revealed that reduced Sema2a levels increased the frequency of ectopic filopodia branching off the TN (top: n = 9 and 6 embryos; p = 0.03) and maximum filopodial length (middle: n = 110 and 131 filopodia; p = 0.001), whereas it decreased the average filopodial life span (bottom: n = 71 and 51 filopodia; p = 0.04). Data are mean ± SEM. E, Frequency of GCaMP5 flashes recorded at a single RP3 bouton in 17e embryos in control and in Sema2a heterozygotes. F, Additional Ca2+ transients do not reduce ectopic frequency in Sema2a/+ heterozygotes. The TrpA1 channels were expressed pan-neurally in Sema2a heterozygotes, and temperature was cycled in a PCR machine following different activation protocols (n = 8, 13, 18, 7, 10, 5, 17, 19, 13, 12, 19, and 10, respectively). *p < 0.05. **p < 0.01. ***p < 0.001.
