Fig. 5.
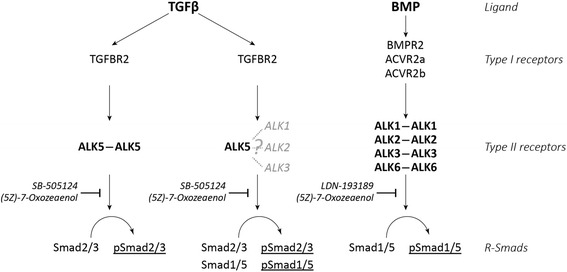
Schematic overview of transforming growth factor β (TGFβ)-induced Smad signaling versus bone morphogenic protein (BMP)-induced Smad signaling in cartilage and the effects of small molecule inhibitors. To signal, a TGFβ dimer binds and induces dimerization of its type II receptor: TGFBR2. This complex subsequently recruits a dimer of the type I receptors TGFβ can bind: activin receptor-like kinase (ALK)1, ALK2, ALK3 and ALK5. Signaling complexes containing ALK5 homodimers will induce Smad2/3 phosphorylation, whereas complexes containing ALK5 combined with ALK1, ALK2 or ALK3 will induce both Smad1/5 and Smad2/3 phosphorylation. However, our current study shows that the kinase domain of ALK1, ALK2 or ALK3 is not involved in TGFβ-induced pSmad1/5 because TGFβ-induced pSmad1/5 is inhibited by SB-505124 but not by LDN-193189. Possibly, ALK1, ALK2 and ALK3 function to recruit Smad1/5 to TGFβ receptor complexes but are not activated by TGFBR2 themselves and are therefore not active. For BMP signaling a heterotetrameric complex is also formed from its different type II and type I receptors; BMPR2, ACVR2a and ACVR2b can be used as type II receptors, whereas ALK1, ALK2, ALK3 and ALK6 function as type I receptors. These complexes will induce pSmad1/5 but this is potently blocked by LDN-193189 (as shown in this article) or partially blocked by (5Z)-7-Oxozeaenol (evidence from the literature)
