Fig. 7.
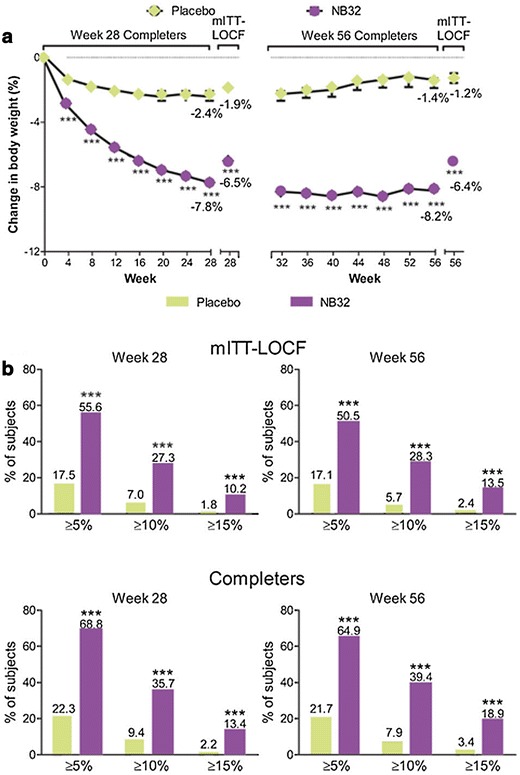
Mean weight loss and categorical weight loss in the COR-II trial. a Percent weight loss (observed; LS mean ± SE) by visit in the week 28 and 56 completers (NB32 data are weighted for weeks 32–56), and percent weight loss for the week 28 and 56 mITT-LOCF subjects. b Categorical weight loss in week 28 and 56 mITT-LOCF and completers. ***P < 0.001 for NB32 vs. placebo. mITT analysis: prespecified modified intent-to-treat population composed of all randomized participants with a baseline weight and ≥1 post-baseline weight on study drug (+1 day post-last dose); LOCF: missing data were imputed by carrying forward the last observation on study drug; completers: participants who completed 28 or 56 weeks of treatment
Adapted from Apovian et al. [62]
