Figure 1.
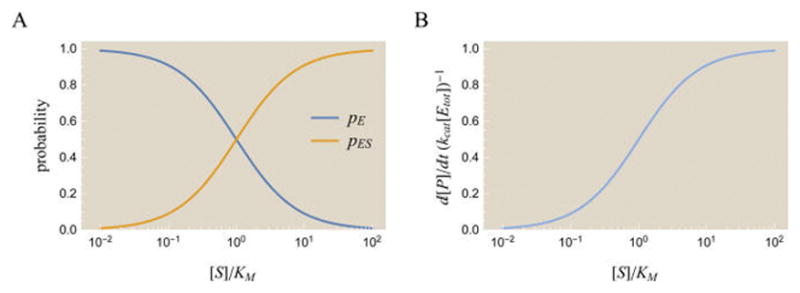
Dynamics of the Michaelis–Menten enzyme. (A) Probabilities of the free enzyme pE and bound enzyme pES states as a function of substrate concentration. As the amount of substrate [S] increases, more enzyme is found in the bound state rather than the free state. (B) The rate of product formation for a nonallosteric enzyme. The rate of product formation has the same functional form as the probability pES of the enzyme– substrate complex, as illustrated by eqs 2 and 7.
