Figure 3.
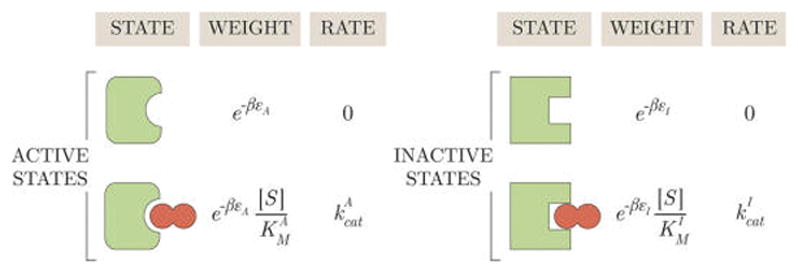
States and weights for a MWC enzyme. The energies εA and εI provide the free energy scale for the substrate-free conformations, dictating their relative probabilities. Decreasing the energy εA of the active state would increase the probability of all the active enzyme conformations relative to the inactive conformations. denotes the substrate concentration at which half of the active enzymes are bound and half of the active enzymes are unbound, as indicated by the crossing of the (pEA, blue) and (pEAS, gold) curves at in Figure 4. serves an analogous role for the inactive states.
