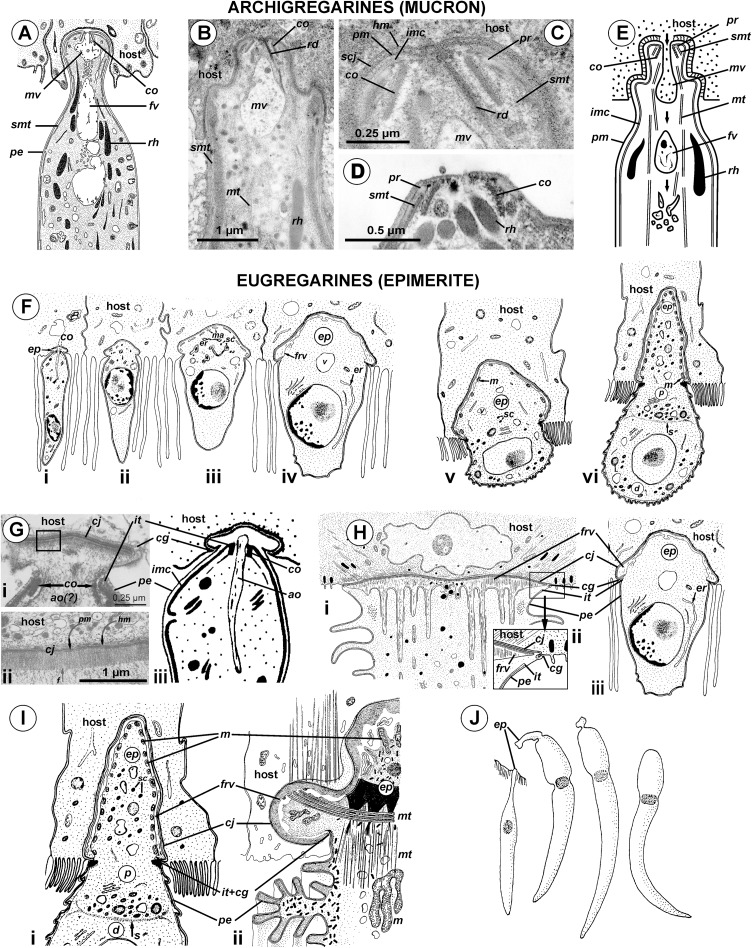
Figure 10. Comparison of the attachment organelles of archigregarines Selenidium spp. (A–E) with septate and aseptate eugregarines (F–I).
(A) Drawing of the apical part of a Selenidium hollandei cell; (B) Ultrastructure of the apical part of an S. orientale cell, a longitudinal section; (C) The frontal region of the mucron under a higher magnification; (D) Mucron of the gamont (syzygy partner) of S. pennatum, a longitudinal section; (E) Predicted myzocytotic feeding in Selenidium; the mucron is embedded in the host cell and contains well-developed apical complex consisting of the conoid (co), polar ring (pr) giving rise to subpellicular microtubules (smt), rhoptries (rh) with rhoptry ducts (rd), and a large mucronal vacuole (mv); the tegument of the mucron comprises a trimembrane pellicle (pe) consisting of the plasma membrane (pm) and internal membrane complex, IMC (imc), with the exception of a small region in front of the conoid, a “cytostome site,” where the IMC is absent and only single plasma membrane is present; the cytostome is intermittently opened in this region to myzocytosis: at first, food comes through the duct (temporary cytopharynx) in the newly formed mucronal vacuole (mv), which then becomes a food vacuole (fv) and is transported into the cell along microtubules (mt) for digestion; the parasite-host contact is mediated by the septate cell junction (scj) with a characteristic wide gap between the plasma membranes (pm and hm, respectively). The mucron with the apical complex persists for a long time into the syzygy; the mucronal food vacuole is absent because the syzygy is a non-feeding stage (D). (F) Development of trophozoite of the septate gregarine Gregarina blaberae (scheme): (i), epimerite (ep) develops as a bulb in front of the apical complex consisting of the conoid and axial organelle (ao), which is likely a homologue of mucronal vacuole (also see (Giii)); the IMC terminates near the apical part of conoid (similarly to mature Selenidium), therefore the developing epimerite is covered only by a single plasma membrane, not by the pellicle; (ii–vi), the apical complex disappears, the epimerite is growing; a large flattened frontal vacuole (frv) arising from the layer of membrane alveoli (ma) of endoplasmic-reticulum (er) origin, numerous mitochondria (m), granules of storage carbohydrate amylopectin (sc), lipid drops (ld), and vacuoles (v) are present in the epimerite cytoplasm; (vi), finally, protomerite (p) and deutomerite (d) are separated by the septum (s). (G) Comparison of developing attachment organelles in the youngest trophozoites of the aseptate gregarine Lecudina sp. from the polychaete Cirriformia (Syn. Audouinia) tentaculata: ((i) and (ii); (ii) shows the details of the cell junction marked by the rectangle in (i)) and G. blaberae ((iii), the magnified fragment of (Fi)): both organelles develop ahead of the conoid in the same way and are covered by a single plasma membrane; the cell junction (cj) between the parasite and host cells is, unlike Selenidium, formed by two closely adjacent plasma membranes (parasite and host); an electron-dense fibrillar zone adjoins the cell junction in the gregarine cell (arrow); the cell junction is bordered by the circular groove (cg) pinching a small portion of the host cell; the IMC terminates (it) at the apical part of the conoid. (H) Comparison of the “mucron” of a well-developed trophozoite of the same Lecudina sp. ((i) and (ii); (ii) is the magnified fragment of (i) marked by the rectangle) and underdeveloped epimerite (ep) of a growing trophozoite of G. blaberae (iii), the same stage as in (Fiv): the IMC terminates (ie) at the base of the attachment organelle (it marks the former apex of the sporozoite mucron), the cell junction consists of two closely adjacent plasma membranes bordered by the circular groove (cg) pinching a small portion of the host cell, a large flattened frontal vacuole (frv) with fibrillar content develops just beneath the region of cell junction. (I) Comparison of the developing epimerite of an older trophozoite of G. blaberae ((i), stage (vi) from (F), magnified) and the attachment organelle of Lecudina (Syn. Cygnicollum) lankesteri (ii); (m), mitochondria. (J) A trophozoite and mature gamonts of L. lankesteri: losing of the epimerite. (A) is reprinted from: Schrével, 1968 (© 1968 Société Française de Microscopie Electronique, Paris), with permission from the Journal de Microscopie et Biology Cellulaire published by Société Française de Microscopie Electronique, Paris; (B, C, and E) are reprinted from: Simdyanov & Kuvardina, 2007 (© 2007 Elsevier), with permission from Elsevier (D) is reprinted from: Kuvardina & Simdyanov, 2002 (© 2002 by Russia, Protistology), with permission from the journal Protistology (Apr 19, 2017); (F, Giii, Hiii, and Ii) are reprinted from: Tronchin & Schrével, 1977 (© 1977 Society of Protozoologists, © John Wiley and Sons), with permission from John Wiley and Sons (Gi, Gii, and Hi) are reprinted from: Ouassi & Porchet-Henneré (1978), with permission from Elsevier #RP016388; (Iii and J) are reprinted from: Desportes & Théodoridès, 1986 (© 1986 Elsevier), with permission from Elsevier #RP016388.