Abstract
Lipid sensor peroxisome proliferator-activated receptor alpha (PPAR-α) is the master regulator of lipid metabolism. Dietary release of endogenous free fatty acids, fibrates, and certain persistent environmental pollutants, e.g. perfluoroalkyl fire-fighting foam components, are peroxisome proliferator-activated receptor alpha ligands. Here, we define a role for peroxisome proliferator-activated receptor alpha in regulating the expression of three ATP-driven drug efflux transporters at the rat and mouse blood–brain barriers: P-glycoprotein (Abcb1), breast cancer resistance protein (Bcrp/Abcg2), and multidrug resistance-associated protein 2 (Mrp2/Abcc2). Exposing isolated rat brain capillaries to linoleic acid, clofibrate, or PKAs increased the transport activity and protein expression of the three ABC transporters. These effects were blocked by the PPAR-α antagonist, GW6471. Dosing rats with 20 mg/kg or 200 mg/kg of clofibrate decreased the brain accumulation of the P-glycoprotein substrate, verapamil, by 50% (in situ brain perfusion; effects blocked by GW6471) and increased P-glycoprotein expression and activity in capillaries ex vivo. Fasting C57Bl/6 wild-type mice for 24 h increased both serum lipids and brain capillary P-glycoprotein transport activity. Fasting did not alter P-glycoprotein activity in PPAR-α knockout mice. These results indicate that hyperlipidemia, lipid-lowering fibrates and exposure to certain fire-fighting foam components activate blood–brain barrier peroxisome proliferator-activated receptor alpha, increase drug efflux transporter expression and reduce drug delivery to the brain.
Keywords: Blood–brain barrier, P-glycoprotein, PPAR, fasting, fibrate
Introduction
Blood–brain barrier (BBB) is a complex network of specialized microvessels with tight intercellular junctions and high expression of plasma membrane drug efflux transporters. The movement of both endogenous and exogenous chemicals to and from the brain is dependent on the BBB. Structural differences between capillary microvessels in the BBB and the periphery make the brain impermeable to most chemicals.1,2 Moreover, the expression of ATP binding cassette efflux transporters (ABC transporters) further hinders passage of xenobiotics from circulation into the brain.3
P-glycoprotein (Pgp/Abcb1), multidrug resistance-associated protein 2 (Mrp2/Abcc2), and breast cancer resistance protein (Bcrp/Abcb11) are major efflux transporters located on the luminal side of brain capillary endothelium. The ability of Pgp and Bcrp to transport a wide range of xenobiotic substrates poses a major challenge for CNS pharmacotherapy. Previous work studying the regulation of these transporters demonstrates that drug exchange across the BBB can be modulated by induction or inhibition of efflux transporters in rodents.4–9 As reviewed by Miller and Cannon,10 efflux transporters at the BBB can be regulated by several stimuli including oxidative stress, inflammatory response, xenobiotic stress, diesel particles, neurodegenerative diseases, and phospholipids. Not only in the BBB, but drug transporter expression in all major organs including liver, kidney, intestine, and placenta is modulated by nuclear transcription factor signaling.11
The peroxisome proliferator-activated receptors (PPARs: α, γ, β/δ) are ligand-activated transcription factors that regulate genes involved in carbohydrate and lipid metabolism, cancer, and inflammation. Elevation of serum levels of free fatty acids, either by a lipid rich diet or fasting-induced adipolysis, activates PPAR-α systemically. For example, after 36 h of fasting, upregulation of fatty acid oxidation-related genes was observed in different regions of brain in male Sprague Dawley rats.12 Xenobiotics also activate PPAR-α. Because of this lipid receptor's role in reducing plasma lipids, PPAR-α agonists, e.g., clofibrate, fenofibrate, fenofibric acid, gemofibrozil, are highly prescribed drugs.13 Owing to lipid oxidation action of PPAR-α, it has been considered as a viable target for non-alcoholic fatty liver disease (NAFLD) therapy.14 The anti-inflammatory actions of PPAR-α make fibrates attractive treatments for neuro-inflammatory disorders, including ischemic stroke, traumatic brain injury, and neurodegenerative diseases.15 In addition, several classes of persistent environmental contaminants activate PPAR-α. These include organic solvents (trichloroethylene), phthalate plasticizers, and perfluoroalkyl (PFA) fire-fighting foam surfactants.16–18
A major target organ for endogenous and xenobiotic PPAR-α ligands is the liver, where PPAR-α regulates expression of a large number of enzymes important in lipid, cholesterol, and bile acid homeostasis. Deficiency of PPAR-α in mice leads to a rise in LDL-cholesterol levels, along with an increase in apolipoprotein B levels.19 Genetic variants of PPAR-α have been detected in human populations; however, their significant impact on the lipid-related disorders was not observed.20 PPAR-α also regulates hepatic genes that code for ABC transporters known to play a critical role in effluxing bile acids and therapeutic drugs. In human hepatoma cells, these include, MDR1, MRP2, MRP3, and MRP4.21–23 At the BBB, our laboratory and those of others have previously demonstrated that the ligand-activated transcription factors, e.g. PXR, CAR, AhR, are capable of inducing expression of all three ABC transporters at the BBB and reducing drug delivery to the brain.6,7,24 A recent study with cultured human brain capillary endothelial cells showed that exposure to clofibrate increased the expression and transport activity of Bcrp,25 suggesting that drug efflux transporters at the BBB may be PPAR-α targets. In this study, we demonstrate that exposure to PPAR-α ligands (lipids, clofibrate, and PFA fire-fighting foam components) increases the transport activity and protein expression of P-glycoprotein, Bcrp and Mrp2 at the BBB.
Materials and methods
Chemicals
The fluorescent P-glycoprotein substrate N-ɛ (4-nitrobenzofurazan-7-yl)-D-Lys8 cyclosporine A (NBD-CSA) was custom synthesized.26 The fluorescent Bcrp substrate BODIPY-Prazosin was purchased from Life Technologies, and fluorescent Mrp2 substrate sulforhodamine 101 free acid (Texas Red) was purchased from Sigma-Aldrich. The specific P-glycoprotein inhibitor, PSC833 (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-1,4,7,10,12,15,19,25,28-nonamethyl-33-[(E,2R)-2-methylhex-4-enoyl]-6,9,18,24-tetrakis (2-methylpropyl) 3,21,30-tri(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31 undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone) was kindly provided by Novartis. Specific Bcrp inhibitor KO143,3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester and specific Mrp2 inhibitor MK571, 5-(3-(2-(7-Chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid sodium salt were ordered from Sigma-Aldrich. PPAR-α agonist clofibrate and antagonist GW6471, N-((2S)-2-(((1Z)-1-Methyl-3-oxo-3-(4-(trifluoromethyl)phenyl)prop-1-enyl)amino)-3-(4-(2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy)phenyl)propyl)propanamide were purchased from Tocris Bioscience. Heptadecafluorooctane sulfonate (PFOS) and heptadecafluorononanoic acid (PFNA) were purchased from Sigma-Aldrich. Mouse polyclonal P-glycoprotein antibody was obtained from Covance, and antibodies against Mrp2 and Bcrp were from Alexis Bio-chemicals. All the reagents used in this study were analytical or best available pharmaceutical grade.
Animals
Animal Care and Use Committee at National Institute of Environmental Health Sciences (ACUC-NIEHS) approved all the animal experiments. All the animal experiments were conducted as per guidelines from ACUC-NIEHS and data are reported in compliance to Animal Research Reporting in Vivo Experiments guidelines (ARRIVE). Retired breeder male Sprague Dawley rats (age ∼9 months; Taconic) were housed under a 12-h light/dark cycle in a temperature controlled facility and allowed access to food and water ad libitum. C57BL/6 and B6; 129S4-PparatmGonz/J (PPAR-α knockout, PPAR-α KO) mice were purchased from The Jackson Laboratory (age: 14 weeks). Animals were killed by CO2 inhalation followed by decapitation. Brain tissue was immediately used for capillary isolation; liver tissue and serum were stored at −80 ℃ until further analysis.
Capillary isolation
For the in vitro experiments (Figures 1, 3 to 5, and 7), brain tissues from 10 rats were pooled and used for capillary isolation and isolated capillaries were divided into desired number of groups for conducting transport assay. Brain capillary microvessels were isolated using the protocol described previously.7 Briefly, brains were homogenized in phosphate-buffered saline (PBS) supplemented with 0.9 mM CaCl2, 0.5 mM MgCl2, 5 mM D-glucose, and 1 mM sodium pyruvate (complete PBS, pH 7.4). The homogenate was mixed with 30% Ficoll solution and centrifuged at 5800g for 20 min at 4 ℃. The vascular pellet was re-suspended in complete PBS with 1% BSA and passed through a glass bead column. Capillaries were segregated by gentle agitation, washed with complete PBS, and then plated in cover glass-bottom chambers for transport assay.
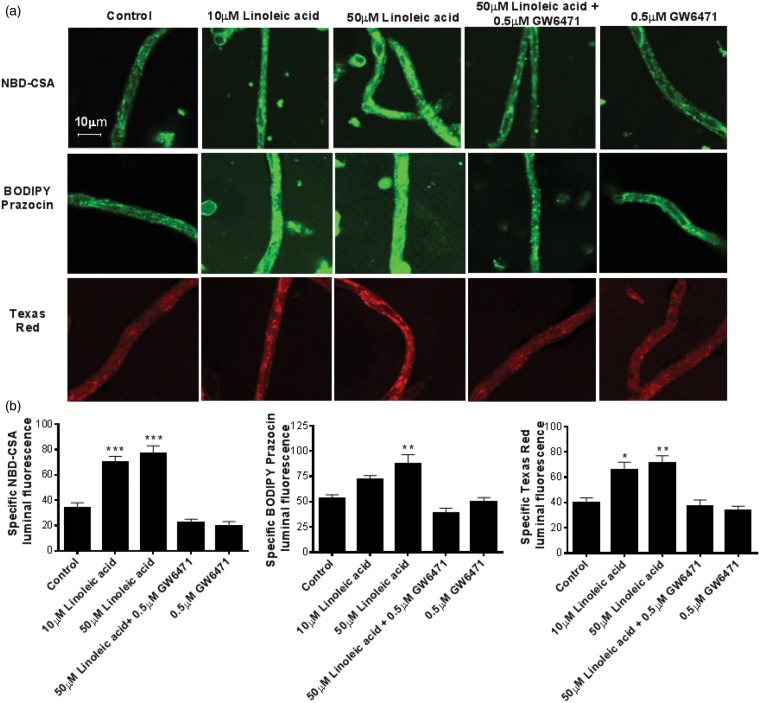
Figure 1.
Linoleic acid upregulates transport activity of P-glycoprotein, Bcrp, and Mrp2 in rat brain capillaries. (a) Representative capillary images. Rat brain capillaries were treated with 10 and 50 μM BSA-conjugated linoleic acid for 4 h, followed by transport assay using fluorescent substrates NBD-CSA (P-glycoprotein), BODIPY Prazosin (Bcrp) and Texas Red (Mrp2). (b) Graphs represent mean fluorescence accumulation ± SEM for minimum of 10 capillaries from each group. All the values were normalized to transporter-specific inhibitor for negative control. Capillaries pre-incubated with GW6471 were used to demonstrate involvement of PPAR-α in transporter induction. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when P < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). ***p < 0.001, significantly higher than control; **p < 0.01, significantly higher than control.
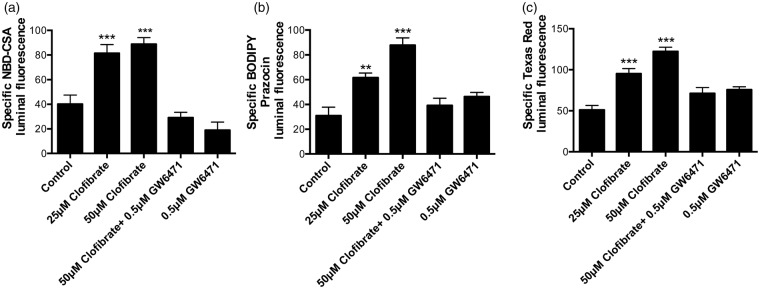
Figure 3.
Clofibrate increases transport activity of P-glycoprotein, Bcrp, and Mrp2 in rat brain capillaries. Rat brain capillaries were treated with 25 and 50 μM clofibrate for 4 h, followed by transport assay using fluorescent substrates. (a) NBD-CSA (P-glycoprotein), (b) BODIPY Prazosin (Bcrp) and (c) Texas Red (Mrp2). Graphs represent mean fluorescence accumulation ± SEM for minimum of 10 capillaries from each group. All the values were normalized to transporter-specific inhibitor for negative control. Capillaries pre-incubated with GW6471 for 45 min were used to demonstrate involvement of PPAR-α in transporter induction. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). ***p < 0.001, significantly higher than control; **p < 0.01, significantly higher than control.
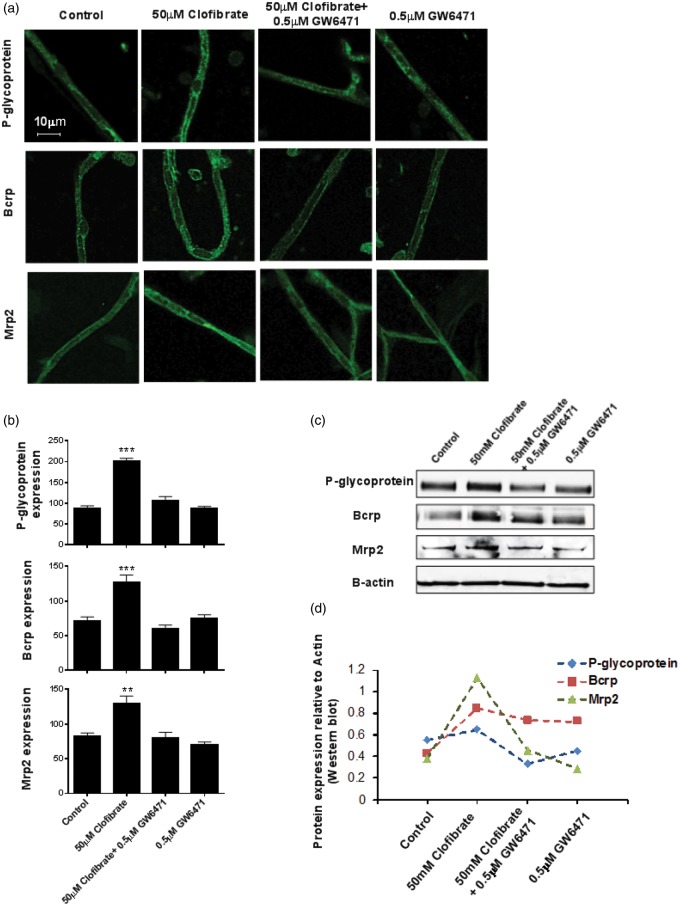
Figure 5.
Clofibrate increases protein expression of P-glycoprotein, Bcrp, and Mrp2 in rat brain capillaries. (a) After exposing rat brain capillaries to 25 and 50 μM clofibrate for 4h, we fixed them with paraformaldehyde-glutaraldehyde mixture, permeabilized with triton X, and subjected to immunohistochemical staining for P-glycoprotein, Bcrp, and Mrp2 using specific primary and fluorescent secondary antibodies. Ten or more confocal images were captured per group and representative images are displayed here. Capillaries pre-incubated with GW6471 for 45 min were used to demonstrate involvement of PPAR-α in transporter induction. (b) Quantification of intensity of membrane fluorescence from the transporter immunostained capillaries, minimum of 10 capillaries per group. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). (c) Membrane fractions were isolated from rat brain capillaries exposed to 25 and 50 μM clofibrate for 4 h. The proteins were separated on SDS-PAGE gels and immunoblotted for P-glycoprotein, Bcrp, and Mrp2 using transporter-specific antibodies. (d) Western blots were quantified and values were normalized to actin expression.
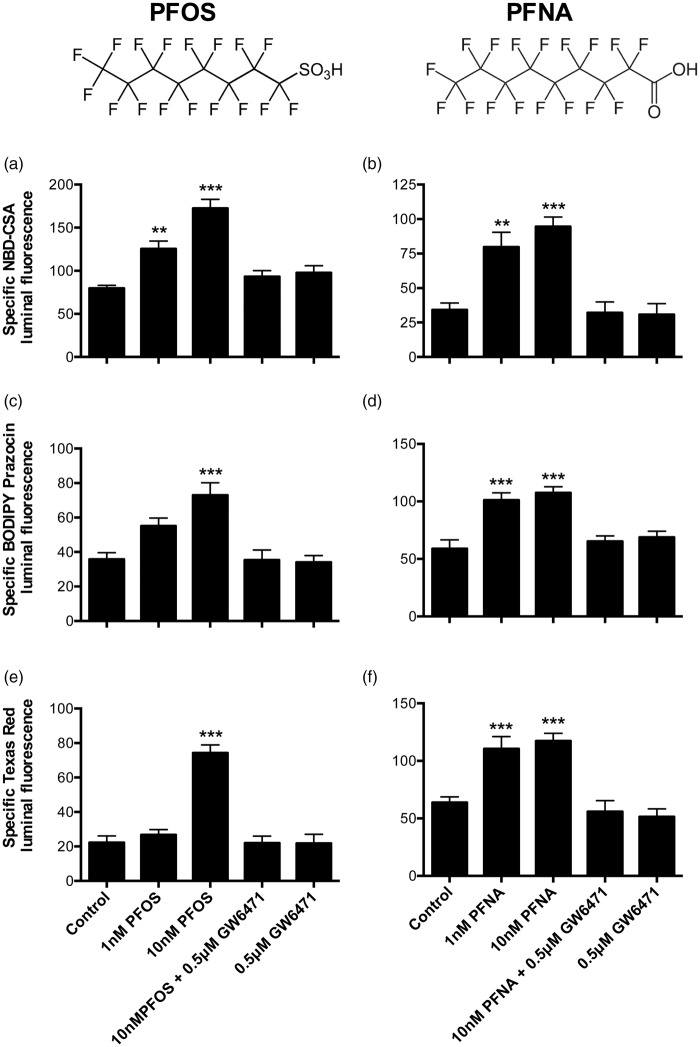
Figure 7.
Perfluorooctane sulfonate (PFOS) and perfluorononanoic acid (PFNA) increase transport activity of P-glycoprotein, Bcrp, and Mrp2 in rat brain capillaries. Rat brain capillaries were treated with 1 and 10 nM PFOS or PFNA for 4 h, followed by transport assay using fluorescent substrates. (a, b) NBD-CSA (P-glycoprotein), (c, d) BODIPY Prazosin (Bcrp), and (e, f) Texas Red (Mrp2). Graphs represent mean fluorescence accumulation ± SEM for minimum of 10 capillaries from each group. All the values were normalized to transporter-specific inhibitor for negative control. Capillaries pre-incubated with GW6471 for 45 min were used to demonstrate involvement of PPAR-α in transporter induction. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). ***p < 0.001, significantly higher than control; **p < 0.01, significantly higher than control.
Transport assay
A detailed method for capillary active transport is described before.3 In summary, the capillaries were plated on glass coverslip bottomed chambers for treatment with the PPAR-α agonists clofibrate or linoleic acid or PFA compound. Some capillaries were pre-treated with the PPAR-α antagonist GW6471 for 45 min before starting the agonist treatment. After 4 h of treatment, chambers received the fluorescent substrate NBD-CSA for P-glycoprotein, BODIPY Prazosin for Bcrp, or Texas Red for Mrp2. After 1 h exposure to fluorescent substrate, the capillaries were assessed for luminal fluorescence accumulation by confocal microscopy (Zeiss 510 Meta laser scanning confocal microscope, 40 × water immersion objective, numerical aperture 1 μM, 488 nm live argon laser for NBD-CSA and BODIPY Prazosin, and 543nM laser for Texas Red). Pre-incubation of capillaries with specific transporter inhibitors provided a negative control for the assay. Luminal fluorescence intensity was measured using ImageJ® software. Specific transport was calculated as the difference in luminal fluorescence between capillaries incubated without and with transporter-specific inhibitor. According to our previously published work,7 time of incubation or isolation does not affect the transport function for P-glycoprotein, for up to 8 h after isolation. Data in Figure 4 (control group) also demonstrate unchanged P-glycoprotein activity for 4 h after isolation. While quantitated as a measure of Mrp2 activity, accumulation of Texas Red also serves as an indicator of capillary integrity for 4 h after isolation (Figure 1).
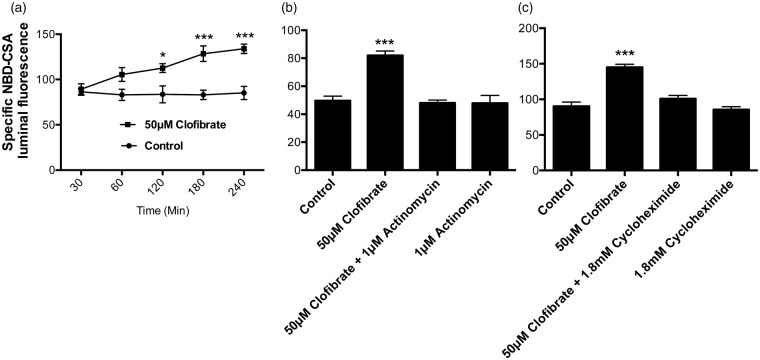
Figure 4.
Clofibrate exposure induces P-glycoprotein transport activity in rat brain capillaries in time and transcription-translation dependent manner. (a) Rat brain capillaries were exposed to clofibrate for 30 to 240 min, followed by P-glycoprotein transport activity assay using fluorescent NBD-CSA. (b) Rat brain capillaries were pre-incubated with transcriptional inhibitor actinomycin or (c) translational inhibitor cycloheximide prior to clofibrate incubation for 4 h. Luminal NBD-CSA accumulation was observed as an indicator of P-glycoprotein transport in these capillaries in vitro. All the data points represent mean fluorescence accumulation ± SEM for minimum of 10 capillaries from each group. The values were normalized to transporter-specific inhibitor PSC833. Capillaries pre-incubated with GW6471 for 45 min were used to demonstrate involvement of PPAR-α in transporter induction. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). ***p < 0.001, significantly higher than control; *p < 0.05, significantly higher than control.
Immunostaining for P-glycoprotein, Bcrp, and Mrp2
Capillary immunohistochemistry was performed as described previously.6 After the treatment, capillaries were fixed with 4% buffered formalin solution containing 0.2% glutaraldehyde for 15 min. After washing, capillaries were permeabilized for 30 min with 1% v/v Triton X-100 solution in PBS. Further, capillaries were blocked with 1% BSA solution for 30 min. Then the capillaries were incubated with anti-P-glycoprotein antibody (C219) or anti-Bcrp antibody (BXP53) or anti-Mrp2 antibody (M2III-6) at 1:100 dilution in PBS overnight at 4 ℃ with shaking. After washing, capillaries were incubated with Alexa Fluor® goat anti-mouse IgG secondary antibody for P-glycoprotein and Mrp2 or Alexa Fluor® goat anti-rat for Bcrp (Life Technologies, Grand Island, NY) diluted 1:1000 in PBS, for 90 min at 37 ℃. Negative controls for the staining were processed without primary antibody, and these only showed background fluorescence. Immunofluorescence was visualized by confocal microscopy (Zeiss LSM 510 meta laser scanning confocal microscope).
Western blot
Membrane proteins were isolated from treated capillaries or liver tissue by protocols described previously.5,6,27 The protein content was quantified by Bradford assay. Membrane proteins were loaded on 4–12% acrylamide-bis NuPAGE gels premixed with NuPAGE 4X sample buffer and NuPAGE reducing agent (Invitrogen, Carlsbad, CA). Proteins were then transferred on Immobilon FL-membrane (Millipore, Billerica, MA) and blocked by Odyssey Blocking buffer (LiCor Biosciences, Lincoln, NE). The membranes were then incubated with transporter-specific primary antibodies as mentioned in the immunostaining procedure, followed by species-specific IRDye in PBS, and imaged using Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
Animal dosing with clofibrate
Two separate dosing regiments were used to demonstrate effects of clofibrate on transport function in vivo. Low dose for 10 days (n=5/group): Male Sprague Dawley rats received oral doses of clofibrate (20 mg/kg per day, in 1% aqueous tween-80 solution) or vehicle for 10 days. Another group was dosed with i.p. GW6471 (1 mg/kg per day, in 1% aqueous methyl cellulose suspension). On the 11th day, brain perfusion was performed as described previously.4
High dose for four days (n=10/group)
Male Sprague Dawley rats received intraperitoneal (i.p.) doses of clofibrate (200 mg/kg per day, in 1% aqueous tween-80 solution) or vehicle for four days. On the 5th day, animals from each group were divided into two subgroups. One subgroup (n=5) was used for capillary isolation for studying transporter activity and expression assay (ex vivo), while the other subgroup (n=5) was used for in situ brain perfusion.
In situ brain perfusion
Rats were anesthetized by i.p. injection with 1 ml/kg ketamine mixture (in mg/ml: 79 ketamine, 3 zylazine, and 0.6 acepromazine) and administered with 10 kU/kg heparin. A midline incision in the neck region was performed to expose the common carotid arteries, which were cannulated and perfused with oxygenated Ringer's solution at 37℃ (117 mM NaCl, 4.7 mM KCl, 0.8 mM MgSO4, 24.8 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 10 mM D-glucose, 39 g/L 170 kDa dextran, and 1 g/L BSA) at 3 ml/min. [14C]Sucrose (0.5 μCi/ml) or [3H] Verapamil (0.1 μCi/ml) were infused into the circuit via syringe pump at 0.5 ml/min for 20 min. Samples of perfusate were collected from the cannulae at the end of each experiment. After perfusion, brains were removed, cleaned of meninges, choroid plexus and midbrain, and solubilized for radioactivity counting. Results were expressed as ratio of disintegrations per minute in the brain/disintegrations per minute in the perfusate (presented as Rbr μl/g).
Fasting wild type and PPAR-α KO mice
C57Bl/6 and PPAR-α KO animals were weighed and transferred to new cages without food and corn bedding. The individually housed animals were allowed ad libitum access to water. Respective control groups were also weighed and allowed access to food and water ad libitum. After 24 h, the animals were anesthetized with isoflurane inhalation, and blood was collected by decapitation. Brains were used for capillary isolation, whereas liver tissue and serum were stored at −80℃ for future use.
Serum free fatty acid quantification
Blood obtained from C57Bl/6 and PPAR-α KO mice was allowed to clot for 30 min on ice, and then spun at 5000 r/min for 10 min to separate serum. Levels of non-esterified free fatty acids (NEFA) were detected in the serum using colorimetric assay from Wako Scientific, as per manufacturer's instructions.
Statistical analyses
Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons) or Student's unpaired t test (GraphPad Prism 5.0c).
Results
PPAR-α activation by fatty acids increases transport activity and protein expression of ABC transporters in rat brain capillaries
Fatty acids are endogenous ligands that activate PPAR-α and induce target gene expression.28 We activated PPAR-α with fatty acids in vitro and in vivo and measured the expression of efflux transporters at the BBB. First, we exposed freshly isolated rat brain capillaries to albumin-conjugated linoleic acid for 4 h and measured specific transport activity of P-glycoprotein, Bcrp, and Mrp2. Figure 1(a) shows representative confocal images of rat brain capillaries exhibiting luminal accumulation of NBD-CSA, a fluorescent substrate for P-glycoprotein. These representative images show that exposure of the capillaries to 10–50 μM linoleic acid increases P-glycoprotein transport activity. These images also show that these increased responses were abolished by the PPAR-α antagonist, GW6471.29
The graphs in Figure 1(b) show the quantitation of capillary luminal fluorescence for P-glycoprotein, Bcrp, and Mrp2 after 4 h exposure to linoleic acid. Exposing freshly isolated capillaries to 10 μM linoleic acid significantly increased transport activity for P-glycoprotein and Mrp2 (Figure 1(b)). In capillaries exposed to 50 μM linoleic acid, P-glycoprotein transport activity more than doubled, Bcrp transport activity increased by 60% and Mrp2 transport activity increased by 80%. Pre-treatment of capillaries for 45 min with 0.5 μM GW6471 prior to linoleic acid exposure blocked the increases in transport activity. GW6471 alone did not affect transport activity of any of the transporters. These data taken together indicate that in rat brain capillaries, linoleic acid increased transport activity of all three ABC transporters in a PPAR-α-dependent manner.
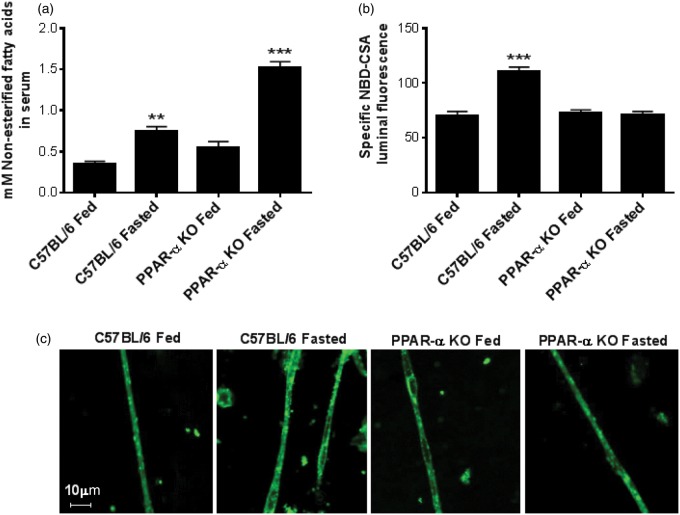
PPAR-α activation in vivo was accomplished by short-term fasting to increase the levels of NEFA in serum.30,31 Specifically, we fasted wild type and PPAR-α KO mice for 24 h and measured NEFA levels in plasma. We also collected brain capillaries from these mice and measured transport function ex vivo. Fasting increased serum NEFA levels two-fold in wild-type mice and three-fold in PPAR-α KO mice (Figure 2(a)). To determine the effect of fatty acid-induced PPAR-α activation in vivo on BBB transport, we measured the P-glycoprotein transport activity in brain capillaries isolated from fasted wild type and fasted PPAR-α KO mice. P-glycoprotein transport activity increased 50% in fasted wild-type mice but remained unchanged in fasted PPAR-α KO mice (Figure 2(b) and (c)), indicating that short-term fasting increases P-glycoprotein transport activity in a PPAR-α dependent manner.
Figure 2.
Effect of 24 h fasting on serum lipids and brain capillary P-glycoprotein function in C57Bl/6 and PPAR-α KO mice. (a) Age and weight matched C57Bl/6 and PPAR-α KO mice (n=5 per group, individually housed) were fasted for 24 h with ad libitum access to water. Serum collected from these mice was used for non-esterified free fatty acid (NEFA) quantification. (b) After 24 h fasting, brains from C57BL/6 and PPAR-α KO mice (n=5 per group) were used for capillary isolation and analyzing P-glycoprotein transport activity ex vivo using fluorescent substrate NBD-CSA. All the values were normalized to P-glycoprotein specific inhibitor PSC833 for negative control. Individual bars in the graph represent mean fluorescence accumulation ± SEM for minimum of 10 capillaries from each group. (c) Representative capillary images from C57BL/6 and PPAR-α KO mice fasting experiment. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). ***p < 0.001, significantly higher than control; **p < 0.01, significantly higher than control.
Induction of efflux transporters in capillaries exposed to clofibrate: a PPAR-α activator
To further investigate the ability of PPAR-α to induce efflux transporters at the BBB we used the drug, clofibrate. Clofibrate is a member of the highly prescribed lipid-lowering fibrate class of drugs (fenofibrate, ciprofibrate, bezafibrate) known to activate PPAR-α. We exposed rat brain capillaries to 25 or 50 μM clofibrate for 4 h and then assayed ABC transporter activity. Figure 3 shows that capillaries exposed to 25 or 50 μM clofibrate more than doubled the transport activity of P-glycoprotein, Bcrp, and Mrp2. Clofibrate-induced increases in transport activity were abolished when the capillaries were pretreated with a PPAR-α antagonist (0.5 μM GW6471).
Next, we investigated the time dependency of the clofibrate-induced increases in P-glycoprotein activity at the BBB. We found that P-glycoprotein transport activity increased significantly after 2 h of clofibrate exposure (Figure 4(a)). Lack of an immediate effect on transport activity suggests that PPAR-α-dependent increases required gene expression. We tested this assumption using inhibitors of transcription (1 μM actinomycin) and translation (1.8 mM cycloheximide). We found that inhibiting transcription or translation blocked the clofibrate-induced increase in P-glycoprotein transport activity (Figure 4(b) and (c)). Neither actinomycin nor cycloheximide alone altered transport activity.
We used immunostaining and Western blotting to measure changes in P-glycoprotein, Bcrp, and Mrp2 protein expression after 4 h exposure of rat brain capillaries to 50 μM clofibrate. Representative confocal images of capillaries immunostained for P-glycoprotein, Bcrp and Mrp2 are shown in Figure 5(a) (quantification of immunostaining in Figure 5(b)). Each image shows the mean plasma membrane fluorescence intensity for capillaries in each treatment group. Exposure to clofibrate significantly increased immunofluorescence of the three transporters. Similarly to previous transport activity assays, clofibrate-induced increases were blocked by GW6471. Western blots from control and clofibrate-treated capillaries also confirmed these results, showing increased protein expression of the three transporters in membranes from capillaries exposed to clofibrate alone. No increases were seen in capillaries exposed to both clofibrate and GW6471 (Figure 5(c) and (d)).
Dosing rats with clofibrate increases P-glycoprotein at the BBB
We used two approaches to determine the effects of clofibrate dosing on P-glycoprotein at the BBB. In the first approach, we dosed rats with clofibrate, isolated capillaries, and measured P-glycoprotein transport activity ex-vivo. When compared to capillaries from vehicle-dosed rats, capillaries from clofibrate-dosed rats exhibited a two-fold increase in transport activity (Figure 6(a)). Capillaries from clofibrate-dosed rats also exhibited increased immunoreactivity for P-glycoprotein (Figure 6(b)).
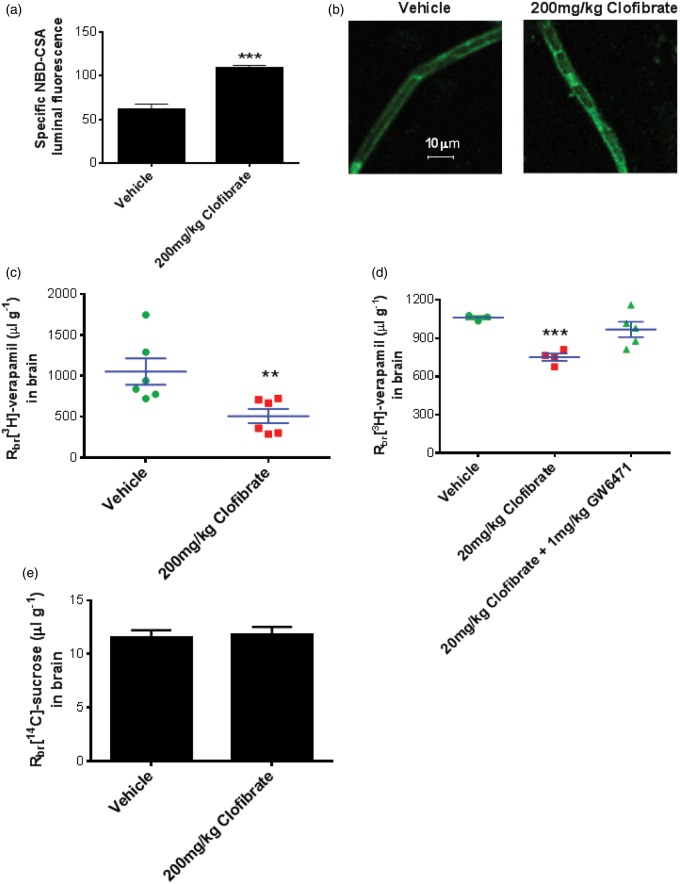
Figure 6.
Clofibrate dosing to rats increases P-glycoprotein activity and expression and reduces drug delivery to the brain. (a) Rats were dosed with 1% aqueous tween-80 (vehicle) or 200 mg/kg clofibrate (i.p., four days, n=5 per group), followed by brain capillary isolation to analyze P-glycoprotein transport activity ex vivo. (b) Capillaries isolated from brains of the rats dosed with 1% aqueous tween-80 (vehicle) or 200 mg/kg clofibrate (i.p., four days, n=5 per group) were subjected to immunohistochemical staining for P-glycoprotein. Representative confocal images are displayed here. (c) After four days of dosing with 1% aqueous tween-80 (vehicle) or 200 mg/kg clofibrate (i.p., four days, n=5 per group), rats were used for in situ brain perfusion. Brain penetration of [3H] verapamil was studied by quantifying radioactivity in brain tissue following in situ perfusion. (d) Rats were dosed with 1% aqueous tween-80 (vehicle) or 20 mg/kg clofibrate or 20 mg/kg clofibrate with 1 mg/kg GW6471 (oral gavage, 10 days, n=5 per group). Rats were subjected to in situ brain perfusion using [3H] verapamil or (E) [14C] sucrose. Data are expressed as mean ± SE. Differences between means were deemed statistically significant when p < 0.05 using one-way ANOVA with a Tukey–Kramer post hoc test (for multiple comparisons). ***p < 0.001, significantly different compared to vehicle; **p < 0.01, significantly different compared to vehicle.
In the second approach, we dosed animals with clofibrate, subjected them to in situ brain perfusion with [3H] verapamil, a P-glycoprotein substrate, and measured brain accumulation of the radiolabeled drug. [3H] Verapamil accumulation in the brain is a sensitive measure of P-glycoprotein activity, with higher accumulation indicating reduced transporter activity and lower accumulation indicating increased activity. Using two different dosing protocols, we observed reduced accumulation of [3H] verapamil in the brains of rats dosed with clofibrate. Dosing with 200 mg/kg clofibrate for four days reduced brain verapamil accumulation by about 50% (Figure 6(c)), while dosing with 20 mg/kg for 10 days reduced [3H] verapamil accumulation by about 30% (Figure 6(d)). Co-administration of 1 mg/kg GW6471 along with clofibrate prevented the clofibrate-induced reduction in [3H] verapamil delivery to brain (Figure 6(d)), indicating the clofibrate effects were PPAR-α dependent. Clofibrate treatment did not affect [14C] sucrose perfusion to brain, indicating intactness of the tight junctions.
PFA persistent organic pollutants increase efflux transport in rat brain capillaries
We extended our investigations to include another important class of PPAR-α activators: PFA fire-fighting foam components and fabric stain repellents.32 Similarly to fatty acids, the structures of these persistent environmental pollutants contain long alkyl chains (Figure 7). We determined the effects of 4 h exposure to 10 or 100 nM heptadecafluorooctane sulfonate (PFOS) and PFNA on transport activity of P-glycoprotein, Bcrp and Mrp2 in rat brain capillaries. Exposing capillaries to 1 nM PFOS increased transport activity of P-glycoprotein but not Bcrp or Mrp2 (Figure 7(a), (c) and (e)). Increasing the PFOS concentration to 10 nM resulted in increased transport activity of all three ABC transporters. PFNA was an even more potent inducer of transport activity, with both 1 and 10 nM causing similar increases for all three transporters (Figure 7(b), (d), and (f)). Note that our initial experiments with 10 nM concentrations of four and six carbon chain PFAs (perfluorobutyric acid and perfluorohexanesulfonic acid) did not show induction of P-glycoprotein activity in rat brain capillaries (data not shown). Thus, it appears that the PFA carbon chain length may be an important determinant of PPAR-α activation. Similar to our observations with linoleic acid and clofibrate, the PPAR-α antagonist GW6471 blocked the effects of PFOS and PFNA on ABC transporter activity (Figure 7).
Discussion
Luminal plasma membrane localization, wide substrate specificity, and high expression levels make P-glycoprotein and Bcrp major obstacles to the delivery of small molecule drugs to the CNS. Reducing transporter activity and/or expression at the BBB increases drug delivery to the CNS. Conversely, increasing transporter activity and/or expression reduces drug delivery to the CNS. Xenobiotic-activated transcription factors coordinately regulate the expression and transport activity of P-glycoprotein, Bcrp, and Mrp2 at the BBB.7,33 These include PXR, CAR, and AhR, all of which translocate from the cytoplasm to the nucleus where they form heterodimers with RXR (PXR and CAR) or ARNT (AhR) before binding to the promoter regions of target genes. Our recent work demonstrated induction of Bcrp function and expression by PPAR-α activation.34
This present study shows that the xenobiotic-activated transcription factor PPAR-α also regulates expression of ABC transporters P-glycoprotein and Mrp2 along with Bcrp at the BBB. We show that diet (lipids and fasting), lipid-lowering drugs (clofibrate) and persistent environmental pollutants (PFAs) increase the transport activity and protein expression of P-glycoprotein, Bcrp and Mrp2 at the BBB in rats and mice. The increases in transporter activity/expression were blocked by a PPAR-α antagonist and in PPAR-α KO mice.
Prior to this study, modulation of BBB transport through diet remained largely unknown. Expression of nutrient-sensitive transcription factors in capillary endothelial cells implicates numerous possibilities that can affect CNS therapy. Along with PPAR-α and expression of PPAR-γ, the carbohydrate response element-binding protein (ChREBP), insulin-like growth factor-1 (Igf-1), and other nutrient responsive factors are also indicated in brain capillary endothelial cells.35–37 This is the first report that demonstrates the impact of short-term fasting on transporter function at the BBB. We utilized C57Bl/6 and PPAR-α KO mice to provide mechanistic evidence that fasting-related induction of P-glycoprotein at the BBB is PPAR-α-dependent. Moreover, transport activity of P-glycoprotein in brain capillaries was not different for C57Bl/6 and PPAR-α KO fed mice, which suggests a role for PPAR-α in inducible transporter regulation as opposed to basal regulation. Our findings also suggest that consuming a diet rich in lipids might also affect CNS therapy through PPAR-α signaling.
PPAR-α dependent increases in ABC transporter expression and activity at the BBB highlights additional considerations for fibrate-based therapies. The transcription factor PPAR-α is a master regulator of fatty acid oxidation in liver and thus is an attractive target for lipid-lowering pharmacotherapy. Indeed, fibrates are among the most highly prescribed classes of drugs in the United States. While clofibrate was discontinued in 2002, fenofibrate, fenofibric acid, and gemofibrozil continue to dominate US markets.13 Fibrates are also prescribed along with statins for the treatment of Type 2 diabetes. In addition, PPAR-α agonists exert anti-inflammatory effects, which can reduce production of cytokines through the modulation of NF-kB.38,39 Moreover, neuro-inflammation plays a key role in the progression of neurological disease, including neurodegenerative diseases.15 In this regard, PPAR-α activation (1) decreased the dopaminergic neuronal loss, and improved behavioral abnormalities in an MPTP-induced model of Parkinsonism,40 (2) attenuated amyloid-β-induced monocyte activation in vitro,41 and (3) reduced effects in an experimental model of spinal cord injury.42 The present results suggest that these potential benefits of PPAR-activation may be accompanied by increased multidrug resistance at the BBB.
In vivo brain perfusion results confirmed that PPAR-α activation at the BBB can reduce brain accumulation of ABC transporter substrates. After oral dosing of rats with a low level of clofibrate for 10 d or i.p. dosing with a higher level for 4 d, P-glycoprotein expression and activity increased and [3H] verapamil accumulation in brain tissue was reduced by 30% and 50%, respectively. At the low clofibrate dose level, co-administration of GW6471, a PPAR-α antagonist, prevented a reduction in brain [3H] verapamil accumulation.
Lastly, our work shows that PFA surfactants, through PPAR-a activation, increase efflux transport in rat brain capillaries. PFAs compounds accumulate in the food chain. Several studies show that human exposure of PFAs levels are in nanomolar range. For example, umbilical cord blood and maternal serum levels of PFOS in pregnant Korean females were between 10 and 40 nM.43 In another study, PFOS levels in breast milk of Spanish women appeared up to 1.7 nM,44 indicating variability in exposure to these chemicals depending on geographical location. Maintaining these relevant human exposure levels, we measured the effect of 1 and 10 nM PFOS and PFNAs on BBB transport function.
Previous findings indicate that persistent organic flame retardant pollutants activate PPAR-α at low nM concentrations.45 Our studies support the notion that structural similarities between PFAs and fatty acids contribute to PPAR-activation. Also, it appears that carbon chain length of the PFA structure is an important determinant for PPAR-α agonistic activity. Our experiments using four and six carbon chain containing PFAs (perfluorobutyric acid, PFBA and perfluorohexanesulfonic acid, PFHxS respectively) did not induce P-glycoprotein function in rat brain capillaries at concentrations similar to PFOS and PFNA (data not shown). Nanomolar levels of PFOS were observed in bodily fluids in normal population.43 Our findings demonstrate the potential of PFOS and PFNA at low concentrations to activate PPAR-α in isolated rat brain capillaries. Exposure levels of 1 nM and 10 nM used in our in vitro assessment are comparable to or lower than occupational exposure of factory workers and firefighters to PFAs.46,47 Literature disagrees about whether the biological effects exerted by PFAs are solely mediated through PPAR-α. Recent evidence also suggests that some PFA effects may occur independently of PPAR-α.48 Our in vitro findings suggest that BBB transport modulation by PFAs is dependent on PPAR-α. Detailed in vivo exposure studies are needed to further understand the impact of these toxicants on BBB function.
Efflux transporters at the BBB, primarily P-glycoprotein, pose major challenges to CNS pharmacotherapy. Our work in rats and mice show that PPAR-α activation increases efflux transport activity at the BBB. Extrapolating this work to humans suggests that PPAR-α activation by fatty acids, fibrates, or flame retardants restricts CNS drug delivery to many patients.
Acknowledgements
We thank Destiny Sykes for excellent technical support and Emily Mesev for fruitful discussions and substantive editing.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
VRM, DSM, and REC designed the project. VRM, CRC, KDO, GNYC, RAE generated the data. VRM, REC, and DSM analyzed the data and contributed to the manuscript preparation.
References
- 1.Crone C, Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol 1981; 77: 349–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 1990; 429: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller DS, Nobmann SN, Gutmann H, et al. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 2000; 58: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab 2010; 30: 1593–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 2004; 66: 387–394. [DOI] [PubMed] [Google Scholar]
- 6.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 2007; 71: 667–675. [DOI] [PubMed] [Google Scholar]
- 7.Bauer B, Hartz AM, Fricker G, et al. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 2004; 66: 413–419. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Campos CR, Peart JC, et al. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci 2014; 34: 8585–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon RE, Peart JC, Hawkins BT, et al. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA 2012; 109: 15930–15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DS, Cannon RE. Signaling pathways that regulate basal ABC transporter activity at the blood-brain barrier. Curr Pharm Des 2014; 20: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 11.Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metabol 2005; 6: 309–328. [DOI] [PubMed] [Google Scholar]
- 12.Konig B, Rauer C, Rosenbaum S, et al. Fasting upregulates PPARalpha target genes in brain and influences pituitary hormone expression in a PPARalpha dependent manner. PPAR Res 2009; 2009: 801609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackevicius CA, Tu JV, Ross JS, et al. Use of fibrates in the United States and Canada. JAMA 2011; 305: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T, Masuzaki H, Nakao K. [Role of PPARs in the pathophysiology of nonalcoholoic fatty liver disease]. Nihon Rinsho 2005; 63: 700–706. [PubMed] [Google Scholar]
- 15.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015; 2015: 610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward JM, Peters JM, Perella CM, et al. Receptor and nonreceptor-mediated organ-specific toxicity of di(2-ethylhexyl)phthalate (DEHP) in peroxisome proliferator-activated receptor alpha-null mice. Toxicol Pathol 1998; 26: 240–246. [DOI] [PubMed] [Google Scholar]
- 17.Vanden HJP, Thompson JT, Frame SR, et al. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 2006; 92: 476–489. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima T, Kamijo Y, Usuda N, et al. Sex-dependent regulation of hepatic peroxisome proliferation in mice by trichloroethylene via peroxisome proliferator-activated receptor alpha (PPARalpha). Carcinogenesis 2000; 21: 677–682. [DOI] [PubMed] [Google Scholar]
- 19.Linden D, Alsterholm M, Wennbo H, et al. PPARalpha deficiency increases secretion and serum levels of apolipoprotein B-containing lipoproteins. J Lipid Res 2001; 42: 1831–1840. [PubMed] [Google Scholar]
- 20.Lacquemant C, Lepretre F, Pineda TI, et al. Mutation screening of the PPARalpha gene in type 2 diabetes associated with coronary heart disease. Diab Metabol 2000; 26: 393–401. [PubMed] [Google Scholar]
- 21.Hossain MA, Tsujita M, Gonzalez FJ, et al. Effects of fibrate drugs on expression of ABCA1 and HDL biogenesis in hepatocytes. J Cardiovasc Pharmacol 2008; 51: 258–266. [DOI] [PubMed] [Google Scholar]
- 22.Kok T, Bloks VW, Wolters H, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha)-mediated regulation of multidrug resistance 2 (Mdr2) expression and function in mice. Biochem J 2003; 369: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher JM, Cheng X, Slitt AL, et al. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metabol Dispos 2005; 33: 956–962. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J 2011; 25: 644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoque MT, Robillard KR, Bendayan R. Regulation of breast cancer resistant protein by peroxisome proliferator-activated receptor alpha in human brain microvessel endothelial cells. Mol Pharmacol 2012; 81: 598–609. [DOI] [PubMed] [Google Scholar]
- 26.Schramm U, Fricker G, Wenger R, et al. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol 1995; 268: F46–F52. [DOI] [PubMed] [Google Scholar]
- 27.More VR, Cheng Q, Donepudi AC, et al. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metabol Dispos 2013; 41: 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bocos C, Gottlicher M, Gearing K, et al. Fatty acid activation of peroxisome proliferator-activated receptor (PPAR). J Steroid Biochem Mol Biol 1995; 53: 467–473. [DOI] [PubMed] [Google Scholar]
- 29.Jeon Y, Jung Y, Kim MC, et al. Sargahydroquinoic acid inhibits TNFalpha-induced AP-1 and NF-kappaB signaling in HaCaT cells through PPARalpha activation. Biochem Biophys Res Commun 2014; 450: 1553–1559. [DOI] [PubMed] [Google Scholar]
- 30.DiMarco NM, Beitz DC, Whitehurst GB. Effect of fasting on free fatty acid, glycerol and cholesterol concentrations in blood plasma and lipoprotein lipase activity in adipose tissue of cattle. J Animal Sci 1981; 52: 75–82. [DOI] [PubMed] [Google Scholar]
- 31.Turcotte LP, Srivastava AK, Chiasson JL. Fasting increases plasma membrane fatty acid-binding protein (FABP(PM)) in red skeletal muscle. Mol Cell Biochem 1997; 166: 153–158. [DOI] [PubMed] [Google Scholar]
- 32.Abbott BD, Wolf CJ, Schmid JE, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci 2007; 98: 571–581. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol 2010; 78: 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoque MT, Shah A, More V, et al. In vivo and ex vivo regulation of breast cancer resistant protein (Bcrp) by peroxisome proliferator-activated receptor alpha (Pparalpha) at the blood-brain barrier. J Neurochem 2015; 135: 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kibbe C, Chen J, Xu G, et al. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J Biol Chem 2013; 288: 23194–23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klotz L, Diehl L, Dani I, et al. Brain endothelial PPARgamma controls inflammation-induced CD4 + T cell adhesion and transmigration in vitro. J Neuroimmunol 2007; 190: 34–43. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Li P, Hua Q, et al. The impact of paracrine signaling in brain microvascular endothelial cells on the survival of neurons. Brain Res 2009; 1287: 28–38. [DOI] [PubMed] [Google Scholar]
- 38.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Therapeut 2006; 110: 371–385. [DOI] [PubMed] [Google Scholar]
- 39.Yang Q, Xie Y, Alexson SE, et al. Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem Pharmacol 2002; 63: 1893–1900. [DOI] [PubMed] [Google Scholar]
- 40.Barbiero JK, Santiago RM, Persike DS, et al. Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Behav Brain Res 2014; 274: 390–399. [DOI] [PubMed] [Google Scholar]
- 41.Combs CK, Bates P, Karlo JC, et al. Regulation of beta-amyloid stimulated proinflammatory responses by peroxisome proliferator-activated receptor alpha. Neurochem Int 2001; 39: 449–457. [DOI] [PubMed] [Google Scholar]
- 42.Esposito E, Rinaldi B, Mazzon E, et al. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR-alpha. J Neuroinflamm 2012; 9: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YJ, Kim MK, Bae J, et al. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere 2013; 90: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 44.Llorca M, Farre M, Pico Y, et al. Infant exposure of perfluorinated compounds: levels in breast milk and commercial baby food. Environ Int 2010; 36: 584–592. [DOI] [PubMed] [Google Scholar]
- 45.Shipley JM, Hurst CH, Tanaka SS, et al. Trans-activation of PPARalpha and induction of PPARalpha target genes by perfluorooctane-based chemicals. Toxicol Sci 2004; 80: 151–160. [DOI] [PubMed] [Google Scholar]
- 46.Fu J, Gao Y, Wang T, et al. Elevated levels of perfluoroalkyl acids in family members of occupationally exposed workers: the importance of dust transfer. Sci Rep 2015; 5: 9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laitinen JA, Koponen J, Koikkalainen J, et al. Firefighters' exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicol Lett 2014; 231: 227–232. [DOI] [PubMed] [Google Scholar]
- 48.Rosen MB, Schmid JR, Corton JC, et al. Gene expression profiling in wild-type and PPARalpha-null mice exposed to perfluorooctane sulfonate reveals PPARalpha-independent effects. PPAR Res 2010; 2010 -. DOI: 10.1155/2010/794739. [DOI] [PMC free article] [PubMed] [Google Scholar]