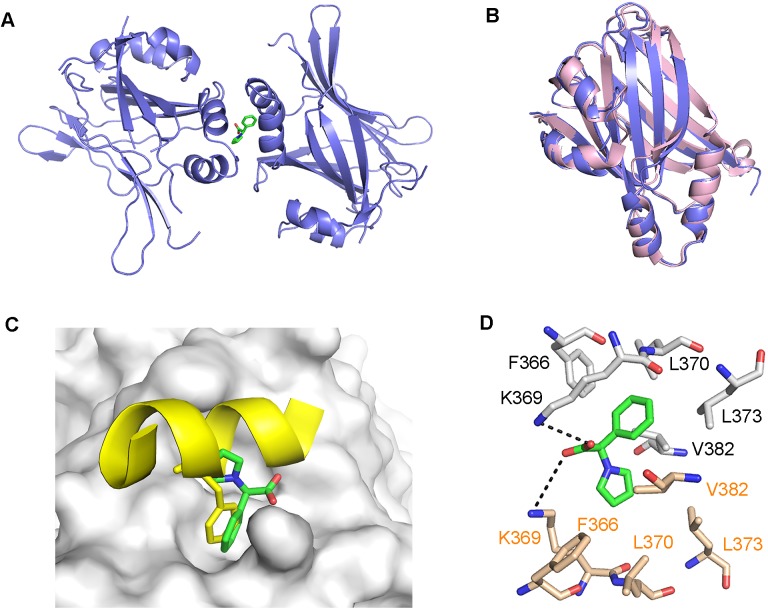
Fig 2. Structure of TEAD-fragment complex.
(a) Overall structure of two TEAD molecules (purple) in complex with hit fragment 1 (green). (b) Overlay of the TEAD molecule from our structure (purple) with that of the human TEAD2 structure (pink, PDB code: 3L15)[39], shows no conformational changes induced by the binding of fragment 1. (c) Overlay of our structure with the mouse TEAD-YAP complex structure (PDB code: 3JUA)[37], reveals that the benzene rings of mYAP Phe54 (yellow sticks) and fragment 1 (green sticks) occupy the same hydrophobic groove on the TEAD surface (grey). (d) Fragment 1 (green sticks) binds to two molecules of TEAD and mainly form hydrophobic interactions with the surface residues of TEAD (grey and beige sticks). The carboxyl group of the fragment also forms hydrogen bonds (black dash) with the side chain of Lys369.